Overview of Recent Advances in Rare-Earth High-Entropy Oxides as Multifunctional Materials for Next-Gen Technology Applications
- PMID: 40076306
- PMCID: PMC11901848
- DOI: 10.3390/molecules30051082
Overview of Recent Advances in Rare-Earth High-Entropy Oxides as Multifunctional Materials for Next-Gen Technology Applications
Abstract
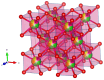
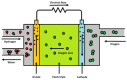
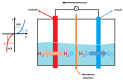
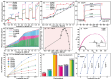
Rare-earth high-entropy oxides are a new promising class of multifunctional materials characterized by their ability to stabilize complex, multi-cationic compositions into single-phase structures through configurational entropy. This feature enables fine-tuning structural properties such as oxygen vacancies, lattice distortions, and defect chemistry, making them promising for advanced technological applications. While initial research primarily focused on their catalytic performance in energy and environmental applications, recent research demonstrated their potential in optoelectronics, photoluminescent materials, and aerospace technologies. Progress in synthesis techniques has provided control over particle morphology, composition, and defect engineering, enhancing their electronic, thermal, and mechanical properties. Rare-earth high-entropy oxides exhibit tunable bandgaps, exceptional thermal stability, and superior resistance to phase degradation, which positions them as next-generation materials. Despite these advances, challenges remain in scaling up production, optimizing compositions for specific applications, and understanding the fundamental mechanisms governing their multifunctionality. This review provides a comprehensive analysis of the recent developments in rare-earth high-entropy oxides as relatively new and still underrated material of the future.
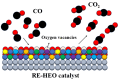
Keywords: CO oxidation; CO2 hydrogenation; catalysis; configuration entropy; high-entropy oxides; hydrogen production; optoelectronics; rare-earth elements; sustainability.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
A review of high-entropy materials with their unique applications.Adv Compos Hybrid Mater. 2025;8(2):195. doi: 10.1007/s42114-025-01275-4. Epub 2025 Mar 3. Adv Compos Hybrid Mater. 2025. PMID: 40046678 Free PMC article. Review.
-
Tuning Oxygen Vacancies in Oxides by Configurational Entropy.ACS Appl Mater Interfaces. 2023 Oct 4;15(39):45774-45789. doi: 10.1021/acsami.3c07268. Epub 2023 Sep 23. ACS Appl Mater Interfaces. 2023. PMID: 37740720
-
Phase Stability and Transitions in High-Entropy Alloys: Insights from Lattice Gas Models, Computational Simulations, and Experimental Validation.Entropy (Basel). 2025 Apr 25;27(5):464. doi: 10.3390/e27050464. Entropy (Basel). 2025. PMID: 40422419 Free PMC article. Review.
-
High-Entropy Oxides: Pioneering the Future of Multifunctional Materials.ACS Nano. 2024 Dec 24;18(51):34492-34530. doi: 10.1021/acsnano.4c12538. Epub 2024 Dec 12. ACS Nano. 2024. PMID: 39666001 Review.
-
Elucidation of the Transport Properties of Calcium-Doped High Entropy Rare Earth Aluminates for Solid Oxide Fuel Cell Applications.Small. 2024 Aug;20(34):e2309735. doi: 10.1002/smll.202309735. Epub 2024 Apr 15. Small. 2024. PMID: 38618655
References
-
- Cantor B., Chang I.T.H., Knight P., Vincent A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A. 2004;375–377:213–218. doi: 10.1016/j.msea.2003.10.257. - DOI
Publication types
LinkOut - more resources
Full Text Sources

