Benefit of an Ultrasonic Irradiation on the Depollution by Washing of Nickel- or Zinc-Contaminated Vermiculite
- PMID: 40076333
- PMCID: PMC11901782
- DOI: 10.3390/molecules30051110
Benefit of an Ultrasonic Irradiation on the Depollution by Washing of Nickel- or Zinc-Contaminated Vermiculite
Abstract
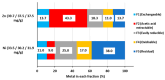
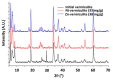
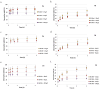
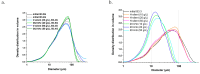
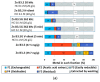
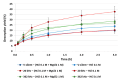
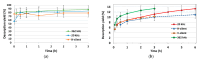
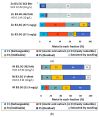
Pollution of soil by heavy metals has become a critical environmental issue. This study investigated an innovative approach to heavy metals removal, focusing on the desorption of nickel and zinc from vermiculite using a combination of leaching and ultrasonic (US) irradiation at 20 or 362 kHz. When 0.1 M HCl was used as a washing solution, Zn2+ desorption yields around 85% were obtained in all conditions. Under 20 kHz US, fragmentation of the particles occurred, leading to the formation of new sites where released Zn2+ could sorb, allowing improved decontamination by cation exchange. Even higher yields were obtained with the biobased citric acid. Ni2+ desorption yields were lower due to its distribution in less accessible Tessier fractions. They significantly increased under US, especially at 362 kHz. It is shown that US leads to transfer of the contaminant from less accessible fractions (in particular the residual one) to more accessible ones, and that at low frequency, new sorption sites are created by fragmentation, leading to readsorption in the exchangeable fraction. This study brought to light for the first time the potential of high-frequency US in enhancing soil washing, to a higher extent compared to low-frequency (20-50 kHz) US.
Keywords: heavy metal; nickel; sequential extraction; soil washing; ultrasound; vermiculite; zinc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Khajuria A., Atienza V.A., Chavanich S., Henning W., Islam I., Kral U., Liu M., Liu X., Murthy I.K., Oyedotun T.D.T., et al. Accelerating Circular Economy Solutions to Achieve the 2030 Agenda for Sustainable Development Goals. Circ. Econ. 2022;1:100001. doi: 10.1016/j.cec.2022.100001. - DOI
-
- Wuana R.A., Okieimen F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011;2011:402647. doi: 10.5402/2011/402647. - DOI
-
- Kaur H., Srivastava S., Goyal N., Walia S. Behavior of Zinc in Soils and Recent Advances on Strategies for Ameliorating Zinc Phyto-Toxicity. Environ. Exp. Bot. 2024;220:105676. doi: 10.1016/j.envexpbot.2024.105676. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

