Bulge-Derived Epithelial Cells Isolated from Human Hair Follicles Using Enzymatic Digestion or Explants Result in Comparable Tissue-Engineered Skin
- PMID: 40076477
- PMCID: PMC11899990
- DOI: 10.3390/ijms26051852
Bulge-Derived Epithelial Cells Isolated from Human Hair Follicles Using Enzymatic Digestion or Explants Result in Comparable Tissue-Engineered Skin
Abstract
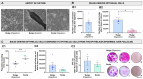
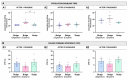
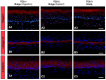
Hair follicle stem cells, located in the bulge region of the outer root sheath, are multipotent epithelial stem cells capable of differentiating into epidermal, sebaceous gland, and hair shaft cells. Efficient culturing of these cells is crucial for advancements in dermatology, regenerative medicine, and skin model development. This investigation aimed to develop a protocol for isolating enriched bulge-derived epithelial cells from scalp specimens to produce tissue-engineered substitutes. The epithelium, including hair follicles, was separated from the dermis using thermolysin, followed by microdissection of the bulge region. Epithelial stem cells were isolated using enzymatic dissociation to create a single-cell suspension and compared with the direct explant culture and a benchmark method which isolates cells from the epidermis and pilosebaceous units. After 8 days of culture, the enzymatic digestion of microdissected bulges yielded 5.3 times more epithelial cells compared to explant cultures and proliferated faster than the benchmark method. Cells cultured from all methods exhibited comparable morphology and growth rates. The fully stratified epidermis of tissue-engineered skin was similar, indicating comparable differentiation potential. This enzymatic digestion method improved early-stage cell recovery and expansion while maintaining keratinocyte functionality, offering an efficient hair bulge cell-extraction technique for tissue engineering and regenerative medicine applications.
Keywords: cell culture technique; hair follicle; primary cell culture; regenerative medicine; stem cells; tissue-engineered skin.
Conflict of interest statement
Pierre Fabre Dermo-Cosmétique, who provided financial support that partially funded this research, had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. Part of the Figure 1 was created with BioRender.com (Agreement number: IT27ODG31G).
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

