Aspirin Eugenol Ester Alleviates Energy Metabolism Disorders by Reducing Oxidative Damage and Inflammation in the Livers of Broilers Under High-Stocking-Density Stress
- PMID: 40076504
- PMCID: PMC11899955
- DOI: 10.3390/ijms26051877
Aspirin Eugenol Ester Alleviates Energy Metabolism Disorders by Reducing Oxidative Damage and Inflammation in the Livers of Broilers Under High-Stocking-Density Stress
Abstract
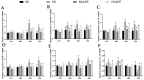
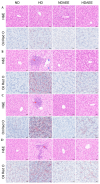
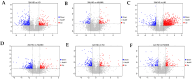
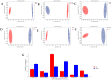
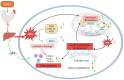
This study aimed to evaluate the effects of aspirin eugenol ester (AEE) on growth performance, oxidative liver damage, inflammation, and liver metabolomics in broilers under high-stocking-density (HSD) stress. A total of 360 broilers were divided into four groups: normal density (ND, 14/m2), high density (HD, 22/m2), ND-AEE (ND + 0.01% AEE), and HD-AEE (HD + 0.01% AEE). HSD decreased total antioxidant capacity, increased malondialdehyde (MDA) levels, and elevated the expression of cyclooxygenase-2 (COX-2) and microsomal prostaglandin E synthase-1 (mPGES-1) mRNA, which contributed to the reduced performance of broilers. Specifically, HSD caused abnormalities in linoleic acid metabolism, leading to elevated levels of Prostaglandin E2 (PGE2) and Leukotriene B4 (LTB4) synthesis, which aggravated inflammation, increased liver lipid levels, and impaired ATP production. AEE counteracted the decline in broiler production performance induced by HSD by enhancing total antioxidant capacity, reducing MDA levels, protecting the liver from oxidative damage, and maintaining mitochondrial oxidative phosphorylation. AEE positively regulated the linoleic acid metabolism by promoting the synthesis of γ-linolenic acid and phosphatidylcholine, which reduced the synthesis of COX-2 and mPGES-1. AEE alleviated the metabolic imbalance caused by HSD stress and enhanced the efficiency of mitochondrial fatty acid oxidation, which reduced excess lipid accumulation in the liver and promoted ATP production. In summary, this study provides strong support for the dietary addition of AEE to alleviate liver oxidative damage, inflammation, and energy metabolism disorders caused by HSD stress.
Keywords: AEE; broilers; energy metabolism; high stocking density; inflammation; oxidative damage.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Effect of dietary aspirin eugenol ester on the growth performance, antioxidant capacity, intestinal inflammation, and cecal microbiota of broilers under high stocking density.Poult Sci. 2024 Jul;103(7):103825. doi: 10.1016/j.psj.2024.103825. Epub 2024 May 11. Poult Sci. 2024. PMID: 38772090 Free PMC article.
-
Effect of dietary supplementation with aspirin eugenol ester on performance and ileum health in broilers under high stocking density stress conditions.Front Vet Sci. 2025 Jul 7;12:1638245. doi: 10.3389/fvets.2025.1638245. eCollection 2025. Front Vet Sci. 2025. PMID: 40693110 Free PMC article.
-
Effects of Aspirin Eugenol Ester on Liver Oxidative Damage and Energy Metabolism in Immune-Stressed Broilers.Antioxidants (Basel). 2024 Mar 13;13(3):341. doi: 10.3390/antiox13030341. Antioxidants (Basel). 2024. PMID: 38539874 Free PMC article.
-
Aspirin Eugenol Ester Modulates the Hypothalamus Transcriptome in Broilers Under High Stocking Density.Animals (Basel). 2025 Mar 13;15(6):823. doi: 10.3390/ani15060823. Animals (Basel). 2025. PMID: 40150351 Free PMC article.
-
Aspirin eugenol ester alleviates lipopolysaccharide-induced acute lung injury in rats while stabilizing serum metabolites levels.Front Immunol. 2022 Jul 29;13:939106. doi: 10.3389/fimmu.2022.939106. eCollection 2022. Front Immunol. 2022. PMID: 35967416 Free PMC article.
References
-
- European Union Council directive 2007/43/EC of 28 June 2007 laying down minimum rules for the protection of chickens kept for meat production. Off. J. Eur. Union. 2007;182:19–28.
MeSH terms
Substances
Grants and funding
- 2022YFE0111100/National Key Research and Development Program of China
- 241111113800/Key Research and Development Program of Henan Province
- 232102521012/Program for International S&T Cooperation Projects of Henan
- 22A230001/Key Scientific Research Foundation of the Higher Education Institutions of Henan Province
- 242102110018/Key Research and Development and Promotion of Special (Science and Technology) Project of Henan Province
LinkOut - more resources
Full Text Sources
Research Materials

