Biological Activity of Peptide Fraction Derived from Hermetia illucens L. (Diptera: Stratiomyidae) Larvae Haemolymph on Gastric Cancer Cells
- PMID: 40076512
- PMCID: PMC11899352
- DOI: 10.3390/ijms26051885
Biological Activity of Peptide Fraction Derived from Hermetia illucens L. (Diptera: Stratiomyidae) Larvae Haemolymph on Gastric Cancer Cells
Abstract
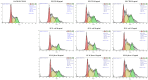
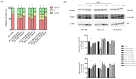
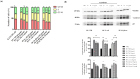
Gastric cancer (GC) is one of the leading causes of cancer-related mortality worldwide, characterised by poor prognosis and limited responsiveness to chemotherapy. There is a need for new and more effective anticancer agents. Antimicrobial peptides (AMPs) represent a promising class of biomolecules for this purpose. Naturally occurring in the innate immune system, these peptides can also exert cytotoxic effects against cancer cells, earning them the designation of "anticancer peptides" (ACPs). They have the potential to be a viable support for current chemotherapy schedules due to their selectivity against cancer cells and minor propensity to induce chemoresistance in cells. Insects are an excellent source of AMPs. Among them, due to its ability to thrive in hostile and microorganism-rich environments, we isolated a peptide fraction from Hermetia illucens L. (Diptera: Stratiomyidae) haemolymph to evaluate a possible anticancer activity. We tested Peptide Fractions (PFs) against AGS and KATO III gastric cancer cell lines. Data obtained indicated that PFs, especially those resulting from Escherichia coli and Micrococcus flavus infection (to boost immune response), were able to inhibit tumour cell growth by inducing apoptosis or cell cycle arrest in a cell line-specific manner. These results support further investigation into the use of antimicrobial peptides produced from insects as possible anticancer agents.
Keywords: anticancer peptides; black soldier fly; haemolymph extracts; in vitro anticancer activity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
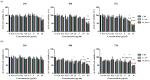



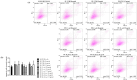
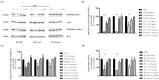

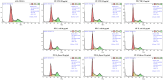
Similar articles
-
Peptide Fractions Extracted from the Hemolymph of Hermetia illucens Inhibit Growth and Motility and Enhance the Effects of Traditional Chemotherapeutics in Human Colorectal Cancer Cells.Int J Mol Sci. 2025 Feb 22;26(5):1891. doi: 10.3390/ijms26051891. Int J Mol Sci. 2025. PMID: 40076518 Free PMC article.
-
In Vitro Evaluation of the Antibacterial Activity of the Peptide Fractions Extracted from the Hemolymph of Hermetia illucens (Diptera: Stratiomyidae).Insects. 2023 May 15;14(5):464. doi: 10.3390/insects14050464. Insects. 2023. PMID: 37233092 Free PMC article.
-
A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae).Sci Rep. 2020 Oct 9;10(1):16875. doi: 10.1038/s41598-020-74017-9. Sci Rep. 2020. PMID: 33037295 Free PMC article.
-
Antimicrobial Peptides from Black Soldier Fly (Hermetia illucens) as Potential Antimicrobial Factors Representing an Alternative to Antibiotics in Livestock Farming.Animals (Basel). 2021 Jun 29;11(7):1937. doi: 10.3390/ani11071937. Animals (Basel). 2021. PMID: 34209689 Free PMC article. Review.
-
No neonates without adults: A review of adult black soldier fly biology, Hermetia illucens (Diptera: Stratiomyidae): A review of adult black soldier fly biology, Hermetia illucens (Diptera: Stratiomyidae).Bioessays. 2023 Jan;45(1):e2200162. doi: 10.1002/bies.202200162. Epub 2022 Nov 16. Bioessays. 2023. PMID: 36382549 Review.
References
-
- National Cancer Institute . ‘Cancer Statistics’, National Institutes of Health. National Cancer Institute; Bethesda, MD, USA: 2024.
-
- Global Cancer Observatory . International Agency for Research on Cancer. World Health Organization; Geneva, Switzerland: 2024.
-
- Global Health Observatory . Explore a World of Health Data. World Health Organization; Geneva, Switzerland: 2024.
-
- World Cancer Research Fund International . Cancer Trends—Worldwide Cancer Data. World Cancer Research Fund International; Sheffield, UK: 2022.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

