Recent Advances on the Role of B Vitamins in Cancer Prevention and Progression
- PMID: 40076592
- PMCID: PMC11900642
- DOI: 10.3390/ijms26051967
Recent Advances on the Role of B Vitamins in Cancer Prevention and Progression
Abstract
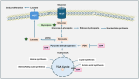
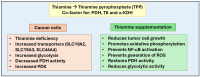
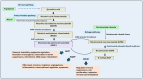
Water-soluble B vitamins, mainly obtained through dietary intake of fruits, vegetables, grains, and dairy products, act as co-factors in various biochemical processes, including DNA synthesis, repair, methylation, and energy metabolism. These vitamins include B1 (Thiamine), B2 (Riboflavin), B3 (Niacin), B5 (Pantothenic Acid), B6 (Pyridoxine), B7 (Biotin), B9 (Folate), and B12 (Cobalamin). Recent studies have shown that besides their fundamental physiological roles, B vitamins influence oncogenic metabolic pathways, including glycolysis (Warburg effect), mitochondrial function, and nucleotide biosynthesis. Although deficiencies in these vitamins are associated with several complications, emerging evidence suggests that excessive intake of specific B vitamins may also contribute to cancer progression and interfere with therapy due to impaired metabolic and genetic functions. This review discusses the tumor-suppressive and tumor-progressive roles of B vitamins in cancer. It also explores the recent evidence on a comprehensive understanding of the relationship between B vitamin metabolism and cancer progression and underscores the need for further research to determine the optimal balance of B vitamin intake for cancer prevention and therapy.
Keywords: B vitamins; Warberg effect; antioxidants; cancer; metabolism; nutrients.
Conflict of interest statement
All the authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

