Impact of Minimally Manipulated Cell Therapy on Immune Responses in Radiation-Induced Skin Wound Healing
- PMID: 40076619
- PMCID: PMC11900442
- DOI: 10.3390/ijms26051994
Impact of Minimally Manipulated Cell Therapy on Immune Responses in Radiation-Induced Skin Wound Healing
Abstract
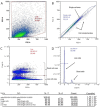
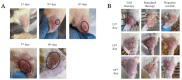
The current treatment of radiation-induced skin wounds utilizes mainly conventional therapies, including topical steroids, creams, ointments, and hydrogel dressings, which do not take into account the immunologic changes that occur in the skin after radiation exposure. Therefore, it is relevant to consider alternative therapies and their impact on changes in the immune landscape of the skin. The aim of this study was to investigate the effect of allogeneic minimally manipulated keratinocytes and fibroblasts on rat skin repair and the development of immune responses. We found that the use of cell therapy compared to treatment with syntazone ointment and no treatment resulted in faster healing and a reduction in the size of radiation-induced skin wounds, area of inflammation, and edema. Additionally, in the group receiving the cell therapy application, there was an observed increase in the number of mast cells (MCs), activation of MC interaction with M2 macrophages, a reduction in the direct contact of MCs with the vascular bed, an increase in the content of collagen fibers due to the intensification of collagen fibrillogenesis, and a restoration of their histotopographic organization. Thus, the positive effect of cell therapy based on allogeneic minimally manipulated keratinocytes and fibroblasts on skin regeneration indicated that it can be used in clinical practice to improve the effectiveness of rehabilitation after radiation therapy.
Keywords: cell therapy; immune responses; ionizing radiation; mast cells; minimally manipulated cells; radiation injury; regenerative medicine; skin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Aliper A.M., Bozdaganyan M.E., Sarkisova V.A., Veviorsky A.P., Ozerov I.V., Orekhov P.S., Korzinkin M.B., Moskalev A., Zhavoronkov A., Osipov A.N. Radioprotectors.org: An open database of known and predicted radioprotectors. Aging. 2020;12:15741–15755. doi: 10.18632/aging.103815. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

