Drug-Induced Liver Injury-Pharmacological Spectrum Among Children
- PMID: 40076629
- PMCID: PMC11901067
- DOI: 10.3390/ijms26052006
Drug-Induced Liver Injury-Pharmacological Spectrum Among Children
Abstract
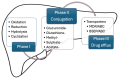
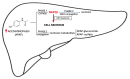
Drug-induced liver injury (DILI) is one of the main causes of acute liver failure in children. Its incidence is probably underestimated, as specific diagnostic tools are currently lacking. Over 1000 known drugs cause DILI, and the list is expanding. The aim of this review is to describe DILI pathogenesis and emphasize the drugs accountable for child DILI in order to aid its recognition. Intrinsic DILI is well described in terms of mechanism, incriminated drugs, and toxic dose. Conversely, idiosyncratic DILI (iDILI) is unpredictable, occurring as a result of a particular response to drug administration, and its occurrence cannot be foreseen in clinical studies. Half of pediatric iDILI cases are linked to antibiotics, mostly amoxicillin-clavulanate, in the immune-allergic group, while autoimmune DILI is the hallmark of minocycline and nitrofurantoin. Secondly, antiepileptics are responsible for 20% of pediatric iDILI cases, children being more prone to iDILI caused by these agents than adults. A similar tendency was observed in anti-tuberculosis drugs, higher incidences being reported in children below three years old. Current data show growing cases of iDILI related to antineoplastic agents, atomoxetine, and albendazole, so that it is advisable for clinicians to maintain a high index of suspicion regarding iDILI.
Keywords: drug metabolism; idiosyncrasy; liver injury; pediatric; toxicity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

