Progress in Plant Nitric Oxide Studies: Implications for Phytopathology and Plant Protection
- PMID: 40076711
- PMCID: PMC11899914
- DOI: 10.3390/ijms26052087
Progress in Plant Nitric Oxide Studies: Implications for Phytopathology and Plant Protection
Abstract
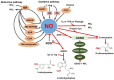
Nitric oxide (NO) is a gaseous free radical known to modulate plant metabolism through crosstalk with phytohormones (especially ABA, SA, JA, and ethylene) and other signaling molecules (ROS, H2S, melatonin), and to regulate gene expression (by influencing DNA methylation and histone acetylation) as well as protein function through post-translational modifications (cysteine S-nitrosation, metal nitrosation, tyrosine nitration, nitroalkylation). Recently, NO has gained attention as a molecule promoting crop resistance to stress conditions. Herein, we review innovations from the NO field and nanotechnology on an up-to-date phytopathological background.
Keywords: nanomaterials; nitric oxide; phytopathogens; plant immunity; stress signaling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Nitric oxide-mediated integrative alterations in plant metabolism to confer abiotic stress tolerance, NO crosstalk with phytohormones and NO-mediated post translational modifications in modulating diverse plant stress.Nitric Oxide. 2018 Feb 28;73:22-38. doi: 10.1016/j.niox.2017.12.005. Epub 2017 Dec 21. Nitric Oxide. 2018. PMID: 29275195 Review.
-
Nitric oxide molecular targets: reprogramming plant development upon stress.J Exp Bot. 2019 Aug 29;70(17):4441-4460. doi: 10.1093/jxb/erz339. J Exp Bot. 2019. PMID: 31327004 Free PMC article. Review.
-
Present knowledge and controversies, deficiencies, and misconceptions on nitric oxide synthesis, sensing, and signaling in plants.Plant Cell Environ. 2020 Jan;43(1). doi: 10.1111/pce.13617. Epub 2019 Aug 22. Plant Cell Environ. 2020. PMID: 31323702 Review.
-
Nitric oxide signaling in the plant nucleus: the function of nitric oxide in chromatin modulation and transcription.J Exp Bot. 2021 Feb 11;72(3):808-818. doi: 10.1093/jxb/eraa404. J Exp Bot. 2021. PMID: 33128375
-
Nitric oxide: An emerging warrior of plant physiology under abiotic stress.Nitric Oxide. 2023 Nov 1;140-141:58-76. doi: 10.1016/j.niox.2023.10.001. Epub 2023 Oct 15. Nitric Oxide. 2023. PMID: 37848156 Review.
Cited by
-
Nitric Oxide and Photosynthesis Interplay in Plant Interactions with Pathogens.Int J Mol Sci. 2025 Jul 20;26(14):6964. doi: 10.3390/ijms26146964. Int J Mol Sci. 2025. PMID: 40725211 Free PMC article. Review.
References
-
- Arasimowicz-Jelonek M., Floryszak-Wieczorek J., Suarez S., Doctorovich F., Sobieszczuk-Nowicka E., Bruce King S., Milczarek G., Rębiś T., Gajewska J., Jagodzik P., et al. Discovery of endogenous nitroxyl as a new redox player in Arabidopsis thaliana. Nat. Plants. 2023;9:36–44. doi: 10.1038/s41477-022-01301-z. - DOI - PMC - PubMed
-
- Kolbert Z., Barroso J.B., Boscari A., Corpas F.J., Gupta K.J., Hancock J.T., Lindermayr C., Palma J.M., Petřivalský M., Wendehenne D., et al. Interorgan, intraorgan and interplant communication mediated by nitric oxide and related species. New Phytol. 2024;244:786–797. doi: 10.1111/nph.20085. - DOI - PubMed
-
- Kumari R., Kapoor P., Mir B.A., Singh M., Parrey Z.A., Rakhra G., Parihar P., Khan M.N., Rakhra G. Unlocking the versatility of nitric oxide in plants and insights into its molecular interplays under biotic and abiotic stress. Nitric Oxide. 2024;150:1–17. doi: 10.1016/j.niox.2024.07.002. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

