Expression Profiles of Five Common Cancer Membrane Protein Antigens Collected for the Development of Cocktail CAR-T Cell Therapies Applicable to Most Solid Cancer Patients
- PMID: 40076777
- PMCID: PMC11900252
- DOI: 10.3390/ijms26052145
Expression Profiles of Five Common Cancer Membrane Protein Antigens Collected for the Development of Cocktail CAR-T Cell Therapies Applicable to Most Solid Cancer Patients
Abstract
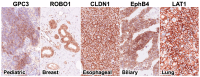
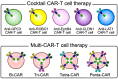
Although CD19 CAR-T has been highly effective against B-cell blood cancers, there are few reports of successful treatments for solid cancers, probably because there are few protein antigens specifically expressed on the surface of the cancer cell membrane. The key to developing a groundbreaking CAR-T cell therapy effective against solid cancers is to "overcome the heterogeneity of cancer antigens". For this purpose, it is necessary to target multiple cancer antigens simultaneously. In this study, we performed immunohistochemical analysis of various solid cancer specimens using antibodies against ROBO1, EphB4, CLDN1, and LAT1 in addition to GPC3, which we have previously studied. These antigens were frequently expressed in various solid cancers but shown to be rarely expressed, with some exceptions, in non-cancerous normal organs adjacent to the cancer. Although ROBO1 and GPC3 are often expressed in cytoplasm, there are also cases in which they are expressed on the cell membrane depending on the type of cancer. On the other hand, it has been revealed that three antigens-EphB4, CLDN1, and LAT1-are frequently expressed only on the cell membrane of cancer cells in various solid cancers, suggesting that they may be ideal targets for CAR-T cell therapy.
Keywords: CAR-T cell therapy; CLDN1; EphB4; LAT1; ROBO1; cocktail CAR-T; common cancer antigens; glypican-3; membrane protein; solid cancer.
Conflict of interest statement
Author Tetsuya Nakatsura (T.N.) has received research grants from BrightPath Biotherapeutics Co., Ltd., Thyas Co., Ltd., ONO PHARMACEUTICAL Co., Ltd., Resonac Corporation, MEDINET Co., Ltd., NapaJen Pharma Inc., Heartseed Inc., Takara BIO Inc., DAICEL CORPORATION, NA Vaccine Institute Co., Ltd., Logomix Inc., Optieum Biotechnologies Inc., and MaxCyte, Inc. T.N. hold stock ownership, stock options, or profits from Noile-Immune Biotech Inc., Logomix Inc., and Optieum Biotechnologies Inc. T.N. have royalties from OncoTherapyScience, Inc. The remaining authors declare that the research was conducted without commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Sawada Y., Yoshikawa T., Nobuoka D., Shirakawa H., Kuronuma T., Motomura Y., Mizuno S., Ishii H., Nakachi K., Konishi M., et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: Immunologic evidence and potential for improving overall survival. Clin. Cancer Res. 2012;18:3686–3696. doi: 10.1158/1078-0432.CCR-11-3044. - DOI - PubMed
-
- Sawada Y., Yoshikawa T., Ofuji K., Yoshimura M., Tsuchiya N., Takahashi M., Nobuoka D., Gotohda N., Takahashi S., Kato Y., et al. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5:e1129483. doi: 10.1080/2162402X.2015.1129483. - DOI - PMC - PubMed
-
- Suzuki S., Sakata J., Utsumi F., Sekiya R., Kajiyama H., Shibata K., Kikkawa F., Nakatsura T. Efficacy of glypican-3-derived peptide vaccine therapy on the survival of patients with refractory ovarian clear cell carcinoma. Oncoimmunology. 2016;5:e1238542. doi: 10.1080/2162402X.2016.1238542. - DOI - PMC - PubMed
-
- Taniguchi M., Mizuno S., Yoshikawa T., Fujinami N., Sugimoto M., Kobayashi S., Takahashi S., Konishi M., Gotohda N., Nakatsura T. Peptide vaccine as an adjuvant therapy for glypican-3-positive hepatocellular carcinoma induces peptide-specific CTLs and improves long prognosis. Cancer Sci. 2020;111:2747–2759. doi: 10.1111/cas.14497. - DOI - PMC - PubMed
-
- Tsuchiya N., Hosono A., Yoshikawa T., Shoda K., Nosaka K., Shimomura M., Hara J., Nitani C., Manabe A., Yoshihara H., et al. Phase I study of glypican-3-derived peptide vaccine therapy for patients with refractory pediatric solid tumors. Oncoimmunology. 2017;7:e1377872. doi: 10.1080/2162402X.2017.1377872. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

