Scutellarin Alleviates Neuronal Apoptosis in Ischemic Stroke via Activation of the PI3K/AKT Signaling Pathway
- PMID: 40076795
- PMCID: PMC11901123
- DOI: 10.3390/ijms26052175
Scutellarin Alleviates Neuronal Apoptosis in Ischemic Stroke via Activation of the PI3K/AKT Signaling Pathway
Abstract
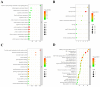
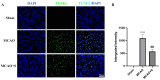
Among all stroke types, ischemic stroke (IS) occurs most frequently, resulting in neuronal death and tissue injury within both the central infarct region and surrounding areas. This study explored the neuroprotective mechanisms of scutellarin, a flavonoid compound, through an integrated strategy that merged in silico analyses (including network pharmacology and molecular docking simulations) with both in vitro and in vivo experimental verification. We identified 1887 IS-related targets and 129 scutellarin targets, with 23 overlapping targets. PPI network analysis revealed five core targets, and molecular docking demonstrated strong binding affinities between scutellarin and these targets. Bioinformatic analyses, including GO functional annotation and KEGG pathway mapping, indicated that the PI3K/AKT cascade represents the primary signaling mechanism. An in vitro experimental system was developed using PC12 cells under oxygen-glucose deprivation conditions to investigate how scutellarin regulates neuronal cell death via the PI3K/AKT pathway. Western blot quantification demonstrated that treatment with scutellarin enhanced the expression of p-PI3K, p-AKT, and Bcl-2 proteins, while simultaneously reducing levels of apoptotic markers Bax and cleaved caspase-3. Furthermore, pharmacological intervention with the selective PI3K inhibitor LY294002 attenuated these molecular alterations, resulting in diminished expression of p-PI3K, p-AKT, and Bcl-2, accompanied by elevated levels of Bax and cleaved caspase-3. In a rat model of middle cerebral artery occlusion, scutellarin administration demonstrated comparable neuroprotective effects, maintaining neuronal survival and modulating apoptotic protein expression via PI3K/AKT pathway activation. Collectively, this study demonstrates the therapeutic potential of scutellarin in cerebral ischemia through PI3K/AKT pathway modulation, suggesting its possible application in treating ischemic disorders.
Keywords: PI3K/AKT pathway; ischemic stroke; molecular docking; network pharmacology; neuronal apoptosis; scutellarin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








Similar articles
-
Integrated network pharmacology, molecular docking and pharmacodynamic study reveals protective effects and mechanisms of corilagin against cerebral ischemia-induced injury.Exp Neurol. 2024 Apr;374:114697. doi: 10.1016/j.expneurol.2024.114697. Epub 2024 Jan 23. Exp Neurol. 2024. PMID: 38266765
-
Scutellarin Modulates Astrocyte-Microglia-Neuron Crosstalk to Mitigate Neuroinflammation and Apoptosis in Cerebral Ischemia.Mol Neurobiol. 2025 Aug;62(8):10834-10855. doi: 10.1007/s12035-025-04933-2. Epub 2025 Apr 15. Mol Neurobiol. 2025. PMID: 40232642
-
The neuroprotective effects of Insulin-Like Growth Factor 1 via the Hippo/YAP signaling pathway are mediated by the PI3K/AKT cascade following cerebral ischemia/reperfusion injury.Brain Res Bull. 2021 Dec;177:373-387. doi: 10.1016/j.brainresbull.2021.10.017. Epub 2021 Oct 28. Brain Res Bull. 2021. PMID: 34717965
-
PI3K/AKT pathway: A potential therapeutic target in cerebral ischemia-reperfusion injury.Eur J Pharmacol. 2025 Jul 5;998:177505. doi: 10.1016/j.ejphar.2025.177505. Epub 2025 Mar 19. Eur J Pharmacol. 2025. PMID: 40118329 Review.
-
Signaling Pathways and Promising Small-Molecule Therapeutic Agents for Ischemic Stroke.ChemMedChem. 2025 May 19;20(10):e202400975. doi: 10.1002/cmdc.202400975. Epub 2025 Mar 27. ChemMedChem. 2025. PMID: 40025810 Review.
References
-
- Wu P., Cheng L.H., Liu Y.L., Zhang J.L., Dong X.M., Chen L., Xu Y.X., Ren Y.Y., Zhang H.M., Liu Z.Q., et al. Elemene mitigates oxidative stress and neuronal apoptosis induced by cerebral ischemia-reperfusion injury through the regulation of glutathione metabolism. J. Ethnopharmacol. 2024;340:119166. doi: 10.1016/j.jep.2024.119166. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

