Hepatic Deletion of Carbohydrate Response Element Binding Protein Impairs Hepatocarcinogenesis in a High-Fat Diet-Induced Mouse Model
- PMID: 40076869
- PMCID: PMC11900174
- DOI: 10.3390/ijms26052246
Hepatic Deletion of Carbohydrate Response Element Binding Protein Impairs Hepatocarcinogenesis in a High-Fat Diet-Induced Mouse Model
Abstract
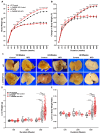
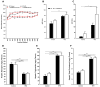
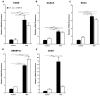
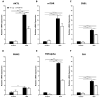
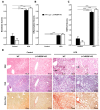
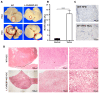
The transcription factor carbohydrate response element binding protein (ChREBP) has emerged as a crucial regulator of hepatic glucose and lipid metabolism. The increased ChREBP activity involves the pro-oncogenic PI3K/AKT/mTOR signaling pathway that induces aberrant lipogenesis, thereby promoting hepatocellular carcinomas (HCC). However, the molecular pathogenesis of ChREBP-related hepatocarcinogenesis remains unexplored in the high-fat diet (HFD)-induced mouse model. Male C57BL/6J (WT) and liver-specific (L)-ChREBP-KO mice were maintained on either a HFD or a control diet for 12, 24, and 48 weeks, starting at the age of 4 weeks. At the end of the feeding period, mice were perfused, and liver tissues were formalin-fixed, paraffin-embedded, sectioned, and stained for histological and immunohistochemical analysis. Biochemical and gene expression analysis were conducted using serum and frozen liver tissue. Mice fed with HFD showed a significant increase (p < 0.05) in body weight from 8 weeks onwards compared to the control. WT and L-ChREBP-KO mice also demonstrated a significant increase (p < 0.05) in liver-to-body weight ratio in the 48-week HFD group. HFD mice exhibited a gradual rise in hepatic lipid accumulation over time, with 24-week mice showing a 20-30% increase in fat content, which further advanced to 80-100% fat accumulation at 48 weeks. Both dietary source and the increased expression of lipogenic pathways at transcriptional and protein levels induced steatosis and steatohepatitis in the HFD group. Moreover, WT mice on a HFD exhibited markedly higher inflammation compared to the L-ChREBP-KO mice. The enhanced lipogenesis, glycolysis, persistent inflammation, and activation of the AKT/mTOR pathway collectively resulted in significant metabolic disturbances, thereby promoting HCC development and progression in WT mice. In contrast, hepatic loss of ChREBP resulted in reduced hepatocyte proliferation in the HFD group, which significantly contributed to the impaired hepatocarcinogenesis and a reduced HCC occurrence in the L-ChREBP-KO mice. Our present study implicates that prolonged HFD feeding contributes to NAFLD/NASH, which in turn progresses to HCC development in WT mice. Collectively, hepatic ChREBP deletion ameliorates hepatic inflammation and metabolic alterations, thereby impairing NASH-driven hepatocarcinogenesis.
Keywords: ChREBP; MLXIPL; NAFLD; NASH; PI3K/AKT/mTOR; fatty liver diseases; hepatocarcinogenesis; hepatocellular carcinoma; high-fat diet; lipogenesis; liver cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mittal S., El-Serag H.B., Sada Y.H., Kanwal F., Duan Z., Temple S., May S.B., Kramer J.R., Richardson P.A., Davila J.A. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans Is Associated with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016;14:124–131.e1. doi: 10.1016/j.cgh.2015.07.019. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

