Unveiling Gestational Diabetes: An Overview of Pathophysiology and Management
- PMID: 40076938
- PMCID: PMC11900321
- DOI: 10.3390/ijms26052320
Unveiling Gestational Diabetes: An Overview of Pathophysiology and Management
Abstract
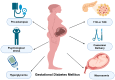
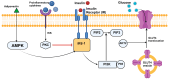
Gestational diabetes mellitus (GDM) is characterized by an inadequate pancreatic β-cell response to pregnancy-induced insulin resistance, resulting in hyperglycemia. The pathophysiology involves reduced incretin hormone secretion and signaling, specifically decreased glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), impairing insulinotropic effects. Pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), impair insulin receptor substrate-1 (IRS-1) phosphorylation, disrupting insulin-mediated glucose uptake. β-cell dysfunction in GDM is associated with decreased pancreatic duodenal homeobox 1 (PDX1) expression, increased endoplasmic reticulum stress markers (CHOP, GRP78), and mitochondrial dysfunction leading to impaired ATP production and reduced glucose-stimulated insulin secretion. Excessive gestational weight gain exacerbates insulin resistance through hyperleptinemia, which downregulates insulin receptor expression via JAK/STAT signaling. Additionally, hypoadiponectinemia decreases AMP-activated protein kinase (AMPK) activation in skeletal muscle, impairing GLUT4 translocation. Placental hormones such as human placental lactogen (hPL) induce lipolysis, increasing circulating free fatty acids which activate protein kinase C, inhibiting insulin signaling. Placental 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) overactivity elevates cortisol levels, which activate glucocorticoid receptors to further reduce insulin sensitivity. GDM diagnostic thresholds (≥92 mg/dL fasting, ≥153 mg/dL post-load) are lower than type 2 diabetes to prevent fetal hyperinsulinemia and macrosomia. Management strategies focus on lifestyle modifications, including dietary carbohydrate restriction and exercise. Pharmacological interventions, such as insulin or metformin, aim to restore AMPK signaling and reduce hepatic glucose output. Emerging therapies, such as glucagon-like peptide-1 receptor (GLP-1R) agonists, show potential in improving glycemic control and reducing inflammation. A mechanistic understanding of GDM pathophysiology is essential for developing targeted therapeutic strategies to prevent both adverse pregnancy outcomes and the progression to overt diabetes in affected women.
Keywords: dietary intervention; gestational diabetes mellitus; glucose intolerance; hyperglycemia; insulin resistance; maternal health; neonatal outcomes; preeclampsia.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Quintanilla Rodriguez B.S., Vadakekut E.S., Mahdy H. StatPearls. StatPearls Publishing LLC.; Treasure Island, FL, USA: 2025. Gestational Diabetes. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

