The Influence of Rare Earth Metals on the Microstructure and Mechanical Properties of 220 and 356.1 Alloys for Automotive Industry
- PMID: 40077168
- PMCID: PMC11901260
- DOI: 10.3390/ma18050941
The Influence of Rare Earth Metals on the Microstructure and Mechanical Properties of 220 and 356.1 Alloys for Automotive Industry
Abstract
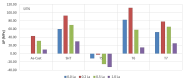
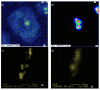
Application of rare earths (RE) as grain refiners is well-known in the technology of aluminum alloys for the automotive industry. In the current study, Al-2.4%Cu-0.4%Mg alloy (coded 220) and Al-7.5%Si-0.35%Mg alloy (coded 356.1), were prepared by melting each alloy in a resistance furnace. Strontium (Sr) was used as a modifier, while titanium boride (TiB2) was added as a grain refiner. Measured amounts of Ce and La were added to both alloys (max. 1 wt.%). The alloy melts were poured in a preheated metallic mold. The main part of the study was conducted on tensile testing at room temperature. The results show that although RE would cause grain refining to be about 30-40% through the constitutional undercooling mechanism, grain refining with TiB2 would lead to approximately 90% refining (heterogenous nucleation mechanism). The addition of high purity Ce or La (99.9% purity) has no modification effect regardless of the alloy composition or the concentration of RE. Depending on the alloy ductility, the addition of 0.2 wt.%RE has a hardening effect that causes precipitation of RE in the form of dispersoids (300-700 nm). However, this increase vanishes with the decrease in alloy ductility, i.e., with T6 treatment, due to intensive precipitation of ultra-fine coherent Mg2Si-phase particles. There is no definite distinction in the behavior of Ce or La in terms of their high affinity to interact with other transition elements in the matrix, particularly Ti, Fe, Cu, and Sr. When the melt was properly degassed using high-purity argon and filtered using a 20 ppi ceramic foam filter, prior to pouring the liquid metal into the mold sprue, no measurable number of RE oxides was observed. In conclusion, the application of RE to aluminum castings would only lead to formation of a significant volume fraction of brittle intermetallics. In Ti-free alloys, identification of Ce- or La-intermetallics is doubtful due to the fairly thin thickness of the precipitated platelets (about 1 µm) and the possibility that most of the reported Al, Si, and other elements make the reported values for RE rather ambiguous.
Keywords: RE; aluminum alloys; automotive industry; grain refining; tensile properties.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures























References
-
- Ford A., Huston D., Cloutier J., Doublier M., Schofield A., Cheng Y., Beyer E. A national scale mineral potential assessment for carbonatite-related rare earth element mineral systems in Australia. Ore Geol. Rev. 2023;161:105658. doi: 10.1016/j.oregeorev.2023.105658. - DOI
-
- Zhou B., Li Z., Chen C. Global potential of rare earth resources and demand for rare earths from clean technologies. Minerals. 2017;7:203. doi: 10.3390/min7110203. - DOI
-
- Long K.R., van Gosen B.S., Foley N.K., Cordier D. The Principle Rare Earth Elements Deposits of the United States—A Summary: Of Domestic Deposits and a Global Perspective. U.S. Geological Survey; Reston, VA, USA: 2010. Scientific Investigations Report 2010-5220.
-
- Shen Y., Jiang Y., Qiu X., Zhao S. Leaching of Light Rare Earth Elements from Sichuan Bastnaesite: A Facile Process to Leach Trivalent Rare Earth Elements Selectively from Tetravalent Cerium. JOM. 2017;69:1976–1981. doi: 10.1007/s11837-017-2458-8. - DOI
-
- Xu D., Shah Z., Cui Y., Jin L., Peng X., Zhang H., Sun G. Recovery of rare earths from nitric acid leach solutions of phosphate ores using solvent extraction with a new amide extractant (TODGA) Hydrometallurgy. 2018;180:132–138. doi: 10.1016/j.hydromet.2018.07.005. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

