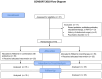
Comparison of Rifaximin Monotherapy and Rifaximin Combined with Probiotics in Patients with Irritable Bowel Syndrome: A Randomized Controlled Trial
- PMID: 40077633
- PMCID: PMC11901931
- DOI: 10.3390/nu17050763
Comparison of Rifaximin Monotherapy and Rifaximin Combined with Probiotics in Patients with Irritable Bowel Syndrome: A Randomized Controlled Trial
Abstract
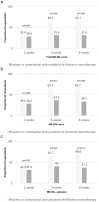
Background/Objective: Rifaximin is a nonabsorbable antibiotic used to treat irritable bowel syndrome (IBS). Recent studies on Helicobacter pylori eradication treatment have reported synergistic effects and low adverse effects when antibiotics are used in combination with probiotics; yet, such studies have not been conducted in IBS. Probiotics can enhance gut microbiota modulation, inhibition of pathogen adhesion to the gut epithelia, improvement in gut barrier function, anti-inflammatory effects, and improvement of gut immunity. Therefore, this study aimed to investigate the efficacy and safety of rifaximin in combination with probiotics compared to rifaximin monotherapy in patients with IBS. Methods: Patients with IBS were randomly allocated to receive rifaximin monotherapy or a combination of rifaximin and probiotics. The primary outcome was the response rate of the total IBS severity scoring system (IBS-SSS) score (>50-point decrease). Secondary outcomes were based on the response rate of the IBS quality of life (IBS-QOL) score and the IBS-SSS1 subscore (>10-point decrease in both scores). Results: Among 70 patients, the responder rates for the total IBS-SSS score were 65.7% in the combination therapy group and 31.4% in the monotherapy group at weeks 4 and 8, respectively (p = 0.004). The responder rates for IBS-QOL were 65.7% versus (vs.) 37.1% and 65.7% vs. 34.2% at weeks 4 and 8, respectively (p = 0.017 and p = 0.009, respectively). The IBS-SSS1 subscore responder rates were 65.7% vs. 40.0% at week 4 and 68.6% vs. 37.1% at 8 weeks (p = 0.031 and p = 0.017, respectively). Conclusions: Rifaximin combined with probiotics was superior to rifaximin monotherapy in patients with IBS. This combination therapy is considered an effective and safe treatment option for patients with IBS. However, further studies are needed to investigate the mechanisms of therapy and long-term outcomes.
Keywords: irritable bowel syndrome; probiotics; randomized controlled trial; rifaximin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical