The major phytocannabinoids, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), affect the function of CatSper calcium channels in human sperm
- PMID: 40078063
- PMCID: PMC12046078
- DOI: 10.1093/humrep/deaf020
The major phytocannabinoids, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), affect the function of CatSper calcium channels in human sperm
Abstract
Study question: Do the main psychoactive phytocannabinoid delta-9-tetrahydrocannabinol (THC) and its non-psychoactive analog cannabidiol (CBD) affect human sperm function?
Summary answer: THC and CBD affect the sperm-specific Ca2+ channel CatSper, suppress activation of the channel by progesterone (P4) and prostaglandin E1 (PGE1), and THC also alters human sperm function in vitro.
What is known already: Marijuana (Cannabis sativa) is one of the most commonly used recreational drugs worldwide. Although the effects of phytocannabinoids on semen parameters have been studied, there is no evidence of a direct impact of THC and CBD on human sperm.
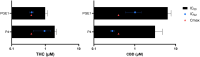
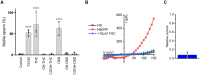
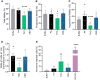
Study design, size, duration: We investigated the effects of the major psychoactive phytocannabinoid, THC, its non-psychoactive analog, CBD, and their major metabolites on Ca2+ influx via CatSper in human spermatozoa. THC and CBD were selected to further evaluate their action on P4-, PGE1-, and pH-induced activation of CatSper. The effects of THC and CBD on sperm motility, penetration into viscous media, and acrosome reaction (AR) were also assessed.
Participants/materials, setting, methods: The effects of phytocannabinoids on CatSper activity were investigated on semen samples from healthy volunteers and men with homozygous deletion of the CATSPER2 gene using kinetic Ca2+ fluorimetry and patch-clamp recordings. Motility was assessed by computer-assisted sperm analysis (CASA). Sperm penetration into viscous media was assessed using a modified Kremer test. The AR was evaluated by flow cytometry using Pisum sativum agglutinin-stained spermatozoa.
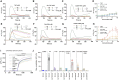
Main results and the role of chance: Both THC and CBD increased the intracellular calcium concentration with CBD inducing a greater increase compared to THC. These Ca2+ signals were abolished in men with homozygous deletion of the CATSPER2 gene demonstrating that they are mediated through CatSper. THC suppressed the P4- and the PGE1-induced Ca2+ increase with a half-maximal inhibitory concentration (IC50) of 1.88 ± 1.15 µM and 0.98 ± 1.10, respectively. CBD also suppressed the P4- and PGE1-induced Ca2+ signal with an IC50 of 2.47 ± 1.12 µM and 6.14 ± 1.08 µM, respectively. The P4 and PGE1 responses were also suppressed by THC and CBD metabolites, yet with greatly reduced potency and/or efficacy. THC and CBD were found to inhibit the Ca2+ influx evoked by intracellular alkalization via NH4Cl, with THC featuring a higher potency compared to CBD. In conclusion, THC and CBD inhibit both the ligand-dependent and -independent activation of CatSper in a dose-dependent manner. This indicates that these phytocannabinoids are genuine CatSper inhibitors rather than P4 and PGE1 antagonists. Finally, THC, but not CBD, impaired sperm hyperactivation and penetration into viscous media and induced a small increase in AR.
Limitations, reasons for caution: Future studies are needed to assess whether cannabis consumption can affect fertility since this study was in vitro.
Wider implications of the findings: The action of THC and CBD on CatSper in human sperm may interfere with the fertilization process, but the impact on fertility remains to be elucidated. THC inhibits the P4 and the PGE1 response more potently than CBD and most previously described CatSper inhibitors. THC can be used as a starting point for the development of non-hormonal contraceptives targeting CatSper.
Study funding/competing interest(s): This work was supported by the Swiss Center for Applied Human Toxicology (SCAHT), the Département de l'Instruction Publique (DIP) of the State of Geneva and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation). The authors declare that no conflicts of interest have been identified that might affect the impartiality of the research reported.
Trial registration number: N/A.
Keywords: CBD; CatSper; THC; acrosome reaction; calcium signaling; cannabis; human sperm; phytocannabinoids; sperm motility.
© The Author(s) 2025. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
The authors declare that no conflict of interest could be perceived as prejudicing the impartiality of the research reported.
Figures


References
-
- Agirregoitia E, Carracedo A, Subirán N, Valdivia A, Agirregoitia N, Peralta L, Velasco G, Irazusta J. The CB2 cannabinoid receptor regulates human sperm cell motility. Fertil Steril 2010;93:1378–1387. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

