Pharmacokinetic effect of disease severity and use of extracorporeal membrane oxygenation in critically ill Asian patients receiving vancomycin
- PMID: 40078297
- PMCID: PMC11897554
- DOI: 10.3389/fphar.2025.1506793
Pharmacokinetic effect of disease severity and use of extracorporeal membrane oxygenation in critically ill Asian patients receiving vancomycin
Abstract
Purpose: Vancomycin is an essential antibiotic for the treatment of severe gram-positive bacterial infections, including methicillin-resistant Staphylococcus aureus (MRSA). In critically ill patients, particularly children, attaining the appropriate dosage is crucial to avert drug resistance and ensure therapeutic efficacy. This study sought to investigate the pharmacokinetics of vancomycin in critically ill Asian pediatric patients and evaluate the influence of extracorporeal membrane oxygenation (ECMO) and disease severity on vancomycin clearance.
Methods: This retrospective analysis examined data from 90 critically ill Asian patients residing in Kaohsiung, Taiwan, encompassing 263 data points gathered over 2 years. A one-compartment pharmacokinetic model with first-order elimination was constructed using nonlinear mixed-effects modeling to assess the impact of ECMO and infection severity on vancomycin clearance.
Results: The pharmacokinetics of vancomycin were markedly affected by ECMO and the severity of the illness. Patients using ECMO demonstrated a 56% decrease in vancomycin clearance relative to non-ECMO patients. Furthermore, patients with milder infections (e.g., cellulitis, surgical prophylaxis, neutropenic fever) had a 39% decrease in vancomycin clearance relative to those with more severe infections (e.g., pneumonia, bacteremia, osteomyelitis, meningitis, deep tissue infection).
Conclusion: The study demonstrates that ECMO and infection severity are major factors influencing vancomycin clearance in critically unwell pediatric patients. The significant decrease in clearance linked to ECMO and reduced infection severity underscores the necessity for meticulous therapeutic drug monitoring and tailored dosing strategies to enhance vancomycin treatment in this at-risk population. The findings highlight the significant interindividual diversity in vancomycin pharmacokinetics in critically unwell pediatric patients.
Keywords: critically ill patients; extracorporeal membrane oxygenation (ECMO); methicillin-resistant Staphylococcus aureus (MRSA); pharmacokinetics; vancomycin.
Copyright © 2025 Avachat, Hung, Birnbaum, Healy, Sherwin and Lin.
Conflict of interest statement
CS is also employed by Differentia Bio; CS holds no stock. The work was completed while CS was at WSU, Dayton, Ohio. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

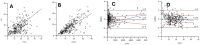

References
-
- Chow T. C. H., Li J. Y. S., Wong J. C. L., Poon F. M. H., Lam H. S., Lam T. T., et al. (2020). Vancomycin prescribing practices and therapeutic drug monitoring for critically ill neonatal and pediatric patients: a survey of physicians and Pharmacists in Hong Kong. Front. Pediatr. 8, 538298. 10.3389/fped.2020.538298 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

