Freshwater faces a warmer and saltier future from headwaters to coasts: climate risks, saltwater intrusion, and biogeochemical chain reactions
- PMID: 40078318
- PMCID: PMC11893707
- DOI: 10.1007/s10533-025-01219-6
Freshwater faces a warmer and saltier future from headwaters to coasts: climate risks, saltwater intrusion, and biogeochemical chain reactions
Abstract
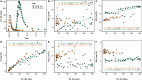
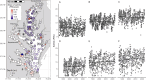
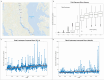
Alongside global climate change, many freshwater ecosystems are experiencing substantial shifts in the concentrations and compositions of salt ions coming from both land and sea. We synthesize a risk framework for anticipating how climate change and increasing salt pollution coming from both land and saltwater intrusion will trigger chain reactions extending from headwaters to tidal waters. Salt ions trigger 'chain reactions,' where chemical products from one biogeochemical reaction influence subsequent reactions and ecosystem responses. Different chain reactions impact drinking water quality, ecosystems, infrastructure, and energy and food production. Risk factors for chain reactions include shifts in salinity sources due to global climate change and amplification of salinity pulses due to the interaction of precipitation variability and human activities. Depending on climate and other factors, salt retention can range from 2 to 90% across watersheds globally. Salt retained in ecosystems interacts with many global biogeochemical cycles along flowpaths and contributes to 'fast' and 'slow' chain reactions associated with temporary acidification and long-term alkalinization of freshwaters, impacts on nutrient cycling, CO2, CH4, N2O, and greenhouse gases, corrosion, fouling, and scaling of infrastructure, deoxygenation, and contaminant mobilization along the freshwater-marine continuum. Salt also impacts the carbon cycle and the quantity and quality of organic matter transported from headwaters to coasts. We identify the double impact of salt pollution from land and saltwater intrusion on a wide range of ecosystem services. Our salinization risk framework is based on analyses of: (1) increasing temporal trends in salinization of tributaries and tidal freshwaters of the Chesapeake Bay and freshening of the Chesapeake Bay mainstem over 40 years due to changes in streamflow, sea level rise, and watershed salt pollution; (2) increasing long-term trends in concentrations and loads of major ions in rivers along the Eastern U.S. and increased riverine exports of major ions to coastal waters sometimes over 100-fold greater than forest reference conditions; (3) varying salt ion concentration-discharge relationships at U.S. Geological Survey (USGS) sites across the U.S.; (4) empirical relationships between specific conductance and Na+, Cl-, SO4 2-, Ca2+, Mg2+, K+, and N at USGS sites across the U.S.; (5) changes in relationships between concentrations of dissolved organic carbon (DOC) and different salt ions at USGS sites across the U.S.; and (6) original salinization experiments demonstrating changes in organic matter composition, mobilization of nutrients and metals, acidification and alkalinization, changes in oxidation-reduction potentials, and deoxygenation in non-tidal and tidal waters. The interaction of human activities and climate change is altering sources, transport, storage, and reactivity of salt ions and chain reactions along the entire freshwater-marine continuum. Our salinization risk framework helps anticipate, prevent, and manage the growing double impact of salt ions from both land and sea on drinking water, human health, ecosystems, aquatic life, infrastructure, agriculture, and energy production.
Supplementary information: The online version contains supplementary material available at 10.1007/s10533-025-01219-6.
Keywords: Anthropogenic salt cycle; Carbon cycle; Climate change; Global biogeochemical cycles; Metals; Nitrogen cycle.
© The Author(s) 2025.
Conflict of interest statement
Competing interestThe authors have no relevant financial or non-financial interests to disclose.
Figures









References
-
- Abolfazli E, Strom K (2021) Deicing road salts may contribute to impairment of streambeds through alterations to sedimentation processes. ACS ES&T Water 2(1):148–155
-
- Acosta JA, Jansen B, Kalbitz K, Faz A, Martínez-Martínez S (2011) Salinity increases mobility of heavy metals in soils. Chemosphere 85(8):1318–1324 - PubMed
-
- Ahmad S (2003) Reinforcement corrosion in concrete structures, its monitoring and service life prediction––a review. Cement Concr Compos 25(4–5):459–471
-
- Aldea CM, Shah SP, Karr A (1999) Effect of cracking on water and chloride permeability of concrete. J Mater Civ Eng 11(3):181–187
-
- Almeida Júnior ES, Martínez A, Gonçalves AL, Canhoto C (2020) Combined effects of freshwater salinization and leaf traits on litter decomposition. Hydrobiologia 847:3427–3435
LinkOut - more resources
Full Text Sources
Miscellaneous
