Effectiveness of Switching from Multiple-Inhaler to Once-Daily Single-Inhaler Triple Therapy in Patients with COPD in a Real-World Setting in Japan
- PMID: 40078928
- PMCID: PMC11899897
- DOI: 10.2147/COPD.S478455
Effectiveness of Switching from Multiple-Inhaler to Once-Daily Single-Inhaler Triple Therapy in Patients with COPD in a Real-World Setting in Japan
Abstract
Purpose: Following the relatively recent introduction of single-inhaler triple therapies in Japan, this study compared the effectiveness of switching from multiple-inhaler triple therapy (MITT) to once-daily fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) by investigating COPD exacerbations and adherence among patients with chronic obstructive pulmonary disease (COPD) in Japan.
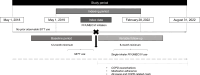
Methods: This retrospective, pre-post cohort study using the Medical Data Vision Co. Ltd database identified patients with ≥1 inpatient diagnosis and/or ≥2 outpatient diagnoses of COPD at age ≥40 years prior to the index date (first/earliest date of single-inhaler FF/UMEC/VI initiation from May 1, 2019-February 28, 2022, following a switch from MITT). The proportion of patients with ≥1 overall (moderate-to-severe), moderate, or severe COPD exacerbation and rate of exacerbations were assessed at 6 months pre- and post-index. Medication adherence (proportion of days covered [PDC]) was also assessed.
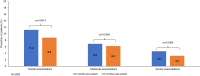
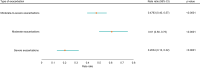
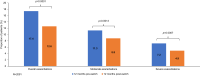
Results: In total, 2365 patients were included, with a mean (standard deviation) age of 75.3 (9.7) years, and 77.1% were male. In the 6 months post-switch from MITT to FF/UMEC/VI, there was a statistically significant decrease in the proportion of patients who experienced ≥1 overall (11.2% to 8.8%; p=0.0014) and severe exacerbation (4.6% to 3.2%; p=0.0069). There was a similar proportion of patients who experienced ≥1 moderate exacerbation pre- and post-switch (6.9% to 6.2%; p=0.2394). Rates of overall (rate ratio [RR]: 0.86, 95% confidence interval [CI]: 0.74-1.00; p=0.0528) and moderate exacerbations (RR: 0.95, 95% CI: 0.79-1.13; p=0.5796) were numerically lower post-switch. There was a significant reduction in severe exacerbations post-switch (RR: 0.68, 95% CI: 0.51-0.90; p=0.0084). Mean PDC was significantly higher in the 6 months post- versus pre-switch (0.83 versus 0.80; p<0.0001).
Conclusion: Patients who switched from MITT to FF/UMEC/VI had reduced exacerbations and improved adherence. These results may help inform healthcare providers on the optimum management strategy for patients with COPD in Japan.
Keywords: adherence; chronic obstructive pulmonary disease exacerbations; claims database; fluticasone furoate/umeclidinium/vilanterol; multiple-inhaler triple therapy.
Plain language summary
Current guidelines recommend that patients with chronic obstructive pulmonary disease (COPD) who experience exacerbations (flare-ups of their symptoms) should be treated with a combination of three treatments, known as triple therapy. Patients can receive multiple-inhaler triple therapy (MITT) or single-inhaler triple therapy (SITT). SITT offers an easier and more convenient treatment plan than MITT and has been shown to improve adherence (the extent to which patients use their treatments as recommended/prescribed). Two SITTs have been approved for use in Japan, including once-daily fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI). Following the relatively recent introduction of SITTs in Japan, this study compared the effectiveness of switching from MITT to FF/UMEC/VI by investigating COPD exacerbations and adherence among patients with COPD in Japan. Patients included in this study were identified from the Medical Data Vision Co. Ltd database, who had switched from using MITT to using FF/UMEC/VI. We assessed COPD exacerbations and adherence to treatment in the 6 months before patients switched from MITT to FF/UMEC/VI and the 6 months following the switch. We found that patients who switched from MITT to FF/UMEC/VI had lower rates of exacerbations in the 6 months after the switch compared with the 6 months before the switch. Patients also had improved treatment adherence in the 6 months post-switch compared with the 6 months pre-switch. The findings of this study may help healthcare providers to prescribe the best treatment plan for patients with COPD in Japan, to help improve their treatment outcomes.
© 2025 Requena et al.
Conflict of interest statement
Gema Requena and Akiko Mizukami were employees of, and/or held financial equities in, GSK at the time of study. Masao Yarita, Kenichi Hashimoto, and Stephen G Noorduyn are employees of, and/or hold financial equities in, GSK. Stephen G Noorduyn is also a PhD candidate at McMaster University. Lucinda J Camidge, Alexander Ford, Thomas Jennison, and Olivia S Massey are employees of Adelphi Real World. Adelphi Real World received funding from GSK to conduct the study only. The authors report no other conflicts of interest in this work.
Figures

References
-
- World Health Organization. Chronic obstructive pulmonary disease (COPD); 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pul.... Accessed January 29, 2024.
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2024. Available from: https://goldcopd.org/2024-gold-report/. Accessed January 29, 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

