Retinoid X receptor agonist 9CDHRA mitigates retinal ganglion cell apoptosis and neuroinflammation in a mouse model of glaucoma
- PMID: 40079201
- PMCID: PMC11904862
- DOI: 10.1096/fj.202402642R
Retinoid X receptor agonist 9CDHRA mitigates retinal ganglion cell apoptosis and neuroinflammation in a mouse model of glaucoma
Abstract
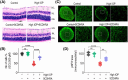
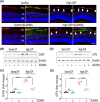
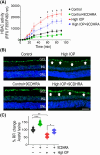
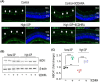
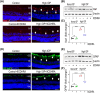
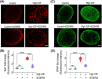
Glaucoma, a leading cause of irreversible blindness, is characterized by the progressive loss of retinal ganglion cells (RGCs) and optic nerve damage, often associated with elevated intraocular pressure (IOP). Retinoid X receptors (RXRs) are ligand-activated transcription factors crucial for neuroprotection, as they regulate gene expression to promote neuronal survival via several biochemical networks and reduce neuroinflammation. This study investigated the therapeutic potential of 9-cis-13,14-dihydroretinoic acid (9CDHRA), an endogenous retinoid RXR agonist, in mitigating RGC degeneration in a high-IOP-induced experimental model of glaucoma. We administered 9CDHRA to glaucomatous mice eyes via intravitreal injections and assessed its effects on endoplasmic reticulum (ER) stress markers, glial cell activation, and RGC survival. Our findings demonstrated that 9CDHRA treatment significantly protected inner retinal function and retinal laminar structure in high-IOP glaucoma. The treatment reduced ER stress markers, increased protein lysine acetylation, and diminished glial cell activation, leading to a significant decrease in apoptotic cells under glaucomatous conditions. These results suggest that 9CDHRA exerts neuroprotective effects by modulating key pathogenic pathways in glaucoma, highlighting its potential as a novel therapeutic strategy for preserving vision in glaucoma.
Keywords: 9CDHRA; ER stress; apoptosis; glaucoma; glial cell; intraocular pressure; neuroinflammation; neuroprotection; retinoid X receptors.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures


References
-
- Gilardi F, Desvergne B. RXRs: Collegial Partners. In: Asson‐Batres MA, Rochette‐Egly C, eds. The Biochemistry of Retinoic Acid Receptors I: Structure, Activation, and Function at the Molecular Level. Springer Netherlands; 2014:75‐102.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

