Electrosynthesis of Atomically Precise Au Nanoclusters
- PMID: 40079235
- PMCID: PMC12061246
- DOI: 10.1002/advs.202414057
Electrosynthesis of Atomically Precise Au Nanoclusters
Abstract
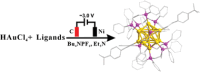
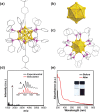
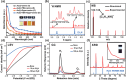
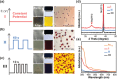
Innovation in synthesis methodologies is crucial for advancing the discovery of new materials. This work reports the electrosynthesis of a [Au13(4-tBuPhC≡C)2(Dppe)5]Cl3 nanocluster (Au13 NC) protected by alkynyl and phosphine ligands. From simple precursor, HAuCl4 and ligands, the whole synthesis is driven by a constant potential in single electrolytic cell. X-ray crystallography determines its total structure. Control experiments, cyclic voltammetry, Proton Nuclear Magnetic Resonance (1H NMR), gas chromatography, and other characterizations demonstrate that a critical tetranuclear Au(I) complex defines the electrochemical redox behavior of the reaction solution. The critical role of a base (e.g., triethylamine) is to suppress the hydrogen evolution reaction at the cathode, paving the way for the reduction of Au ions. To resolve the problem of over-reduction and deposition of Au on the cathode, pulsed electrolysis, which is specific to electrosynthesis is employed. It significantly improves the reaction rate and the isolated yield of Au13. To extend the application scope, another four NCs protected by different ligands, [Au13(4-FPhC≡C)2(Dppe)5]Cl3, [Au8(2-CF3PhC≡C)2(Dppp)4](PF6)2, [Au11(Dppp)5]Cl3, and [Au8(SC2H4Ph)2(Dppp)4]Cl2 are synthesized electrochemically, demonstrating the versatility of the strategy.
Keywords: Au nanoclusters; electrosynthesis; pulsed electrolysis.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Leech M. C., Lam K., Nat. Rev. Chem. 2022, 6, 275. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
