Nonstructural Protein 1 of Influenza A (NS1A) Demonstrates Strain-Specific dsRNA Binding Capabilities
- PMID: 40079391
- PMCID: PMC11997982
- DOI: 10.1021/acsinfecdis.4c00882
Nonstructural Protein 1 of Influenza A (NS1A) Demonstrates Strain-Specific dsRNA Binding Capabilities
Abstract
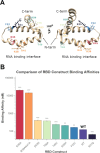
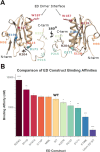
Nonstructural protein 1 of influenza A (NS1A) is a key virulence factor produced inside host cells infected with Influenza A Virus (IAV) and consists of an N-terminal dsRNA binding domain (RBD) and a C-terminal effector domain (ED), joined by a flexible linker. While NS1A is a highly promiscuous protein with a number of intracellular functions, its primary function is nonspecific dsRNA binding that enables influenza to evade our innate immune system. For this reason, NS1A has long been proposed as a potential drug target. Previous research in the field has demonstrated the necessity of dimer formation through the RBD to enable dsRNA binding, which is further enhanced by oligomerization through ED interactions. However, there has been minimal exploration of potential strain-specific effects on dsRNA binding. Most existing studies are limited to the A/Udorn/307/1972 strain, often with a C-terminal tail deletion. Here we utilize fluorescence polarization (FP) paired with fluorescence-based electrophoretic mobility shift assays (fEMSA) to characterize the dsRNA binding properties of NS1A from the H1N1 strain responsible for the 1918 "Spanish Flu" with an intact C-terminal tail. We show that A/Brevig Mission/1/1918 NS1A contains specific residues in the RBD that enhance dsRNA binding. We further demonstrate that both Brevig Mission and Udorn NS1A bind directly to dsRNA through the highly basic C-terminal tail of the ED. These novel binding interactions may have contributed to the increased pathogenicity of the 1918 flu pandemic and may have implications for NS1A-targeted antivirals.
Keywords: H1N1; H3N2; Influenza A; Spanish Flu; dsRNA.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- CDC . Disease Burden of Influenza https://www.cdc.gov/flu/about/burden/index.html (accessed Jul 25, 2024).
-
- Iuliano D.; Roguski K. M.; Chang H. H.; Palekar R.; Tempia S.; Cohen C.; Gran M.; Schanzer D.; Cowling P. B. J.; Wu P.; Kyncl J.; Ang L. W.; Park M.; Redlberger-fritz M.; Espenhain L.; Krishnan P. A.; Emukule G.; Asten L. Van; Aungkulanon S.; Widdowson M.; Bresee J. S. Estimates of Global Seasonal Influenza-Associated Respiratory Mortality: A Modelling Study. Lancet 2018, 391 (10127), 1285–1300. 10.1016/S0140-6736(17)33293-2. - DOI - PMC - PubMed
-
- CDC . Vaccine Effectiveness: How Well do the Flu Vaccines Work? https://www.cdc.gov/flu/vaccines-work/vaccineeffect.htm (accessed Jul 25, 2024).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

