Flagellar Assembly Factor FliW2 De-Represses Helicobacter pylori FlaA-Mediated Motility by Allosteric Obstruction of Global Regulator CsrA
- PMID: 40079448
- PMCID: PMC11905337
- DOI: 10.1111/hel.70019
Flagellar Assembly Factor FliW2 De-Represses Helicobacter pylori FlaA-Mediated Motility by Allosteric Obstruction of Global Regulator CsrA
Abstract
Background: Helicobacter pylori colonizes the human stomach as a dominant member of the gastric microbiota and constitutively expresses flagellar motility for survival. Carbon storage regulator A (CsrA) is a posttranscriptional global regulator and a critical determinant of H. pylori's motility and pathogenicity. The regulation of H. pylori CsrA is still uncertain although in other species CsrA is reported to be antagonized by small RNAs and proteins. In this study, we attempted to unveil how CsrA is regulated and hypothesized that H. pylori CsrA activity is antagonized by a flagellar assembly factor, FliW2, via protein allosteric obstruction.
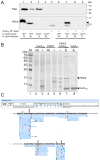
Materials and methods: Multiple sequence comparisons indicated that, along its length and in contrast to fliW1, the fliW2 of H. pylori J99 is conserved. We then generated an isogenic ΔfliW2 strain whose function was characterized using phenotypic and biochemical approaches. We also applied a machine learning approach (AlphaFold2) to predict FliW2-CsrA binding domains and investigated the FliW2-CsrA interaction using pull-down assays and in vivo bacterial two-hybrid systems.
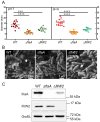
Results: We observed the reduced expression of major flagellin FlaA and impaired flagellar filaments that attenuated the motility of the ΔfliW2 strain. Furthermore, a direct interaction between FliW2 and CsrA was demonstrated, and a novel region of the C-terminal extension of CsrA was suggested to be crucial for CsrA interacting with FliW2. Based on our AlphaFold2 prediction, this C-terminal region of FliW2-CsrA interaction does not overlap with CsrA's N-terminal RNA binding domain, implying that FliW2 allosterically antagonizes CsrA activity and restricts CsrA's binding to flaA mRNAs.
Conclusions: Our data points to novel regulatory roles that the H. pylori flagellar assembly factor FliW2 has in obstructing CsrA activity, and thus FliW2 may indirectly antagonize CsrA's regulation of flaA mRNA processing and translation. Our findings reveal a new regulatory mechanism of flagellar motility in H. pylori.
Keywords: Helicobacter pylori; CsrA; FliW2; flagellar biosynthesis; major flagellin a (FlaA); motility.
© 2025 The Author(s). Helicobacter published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Lei C., Xu Y., Zhang S., Huang C., and Qin J., “The Role of Microbiota in Gastric Cancer: A Comprehensive Review,” Helicobacter 29, no. 2 (2024): e13071. - PubMed
-
- Singh H., Ye A., and Ferrua M. J., “Aspects of Food Structures in the Digestive Tract,” Current Opinion in Food Science 3 (2015): 85–93.
MeSH terms
Substances
Grants and funding
- 111-2811-B-468-001/National Science and Technology Council in Taiwan
- 112-2811-B-468-001/National Science and Technology Council in Taiwan
- 113-2811-B-468-002/National Science and Technology Council in Taiwan
- 108-2320-B-010-002/ministry of science and technology in Taiwan
- 108-2811-B-010-536/ministry of science and technology in Taiwan
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

