Phytochemical, In Silico, In Vitro, and In Vivo Research on Piptadeniastrum africanum (Fabaceae) Unveiling Anti-Stereotypic, Anxiolytic, and Analgesic Effects in a Sodium Valproate-Induced Autistic Disorders Model
- PMID: 40079500
- PMCID: PMC11904952
- DOI: 10.1002/brb3.70408
Phytochemical, In Silico, In Vitro, and In Vivo Research on Piptadeniastrum africanum (Fabaceae) Unveiling Anti-Stereotypic, Anxiolytic, and Analgesic Effects in a Sodium Valproate-Induced Autistic Disorders Model
Abstract
Objective: Individuals with autistic spectrum disorders (ASD) primarily exhibit deficits in communication and social interaction, along with repetitive behaviors and restricted interests. This disorder is often associated with anxiety, nociceptive disorders, and pain. While medical treatment generally focuses on treating the symptoms rather than addressing the underlying causes, traditional medicine is sometimes used as an alternative. Piptadeniastrum africanum is used in Cameroonian medicinal folks to treat cognitive disorders. However, its effects and mechanisms of action regarding the inhibition of ASD-like symptoms remain unclear. The primary goal of the present study was to evaluate the anxiolytic and analgesic effects of the water extract of P. africanum on autistic triad induced in rats by sodium valproate.
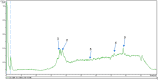
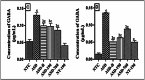
Material and methods: The study investigated the secondary metabolites in P. africanum extract using UHPLC-MS. DPPH, ABTS, and FRAP tests were performed to assess the extract's ability to neutralize free radicals. Molecular docking was utilized to evaluate the extract's binding to various receptors. For the experimental study, 33 pregnant female rats were divided into two groups after pregnancy was confirmed. One group was given distilled water orally at 10 mL/kg, while the other group received sodium valproate at 800 mg/kg on gestation days 11, 12, and 13. When the male offspring reached 3 weeks old, they were evaluated for anxiety, social interaction, and pain sensitivity, with those displaying any disorders selected for further study. The remaining rats were split into six groups of five and treated with either a vehicle, bumetanide, or P. africanum extract at 190 and 760 mg/kg. Behavioral assessments focusing on sociability, anxiety, and pain sensitivity were conducted on days 28 and 37 after weaning. In the end, biochemical markers related to GABA metabolism, serotonin levels, and oxidative status were analyzed in the cerebellum, prefrontal cortex, hippocampus, and amygdala alongside histopathological analyses in the brain.
Results: UHPLC-MS allows us to identify several compounds. They bind to H3R (7F61) and HDAC2 through conventional hydrogen bonding. Findings showed that prenatal administration of sodium valproate induced in male offspring a deficit in social interaction (p < 0.001), anxiety disorders (p < 0.001), hypersensitivity to pain (p < 0.001), increased GABA and serotonin concentration (p < 0.001), disturbed oxidative status (p < 0.001), and neuronal loss (p < 0.001) as well as neuronal disorganization in the hippocampus, cerebellum and amygdala in young rats compared to neurotypical animals. P. africanum extract at doses used, like bumetanide, corrected these disorders and protected against neuronal loss. These results suggest that the extract has anxiolytic and anti-nociceptive effects. It has been found that the positive effects can be achieved by restoring GABAergic and serotonergic neurotransmission, coupled with antioxidant and neuromodulatory activity.
Conclusion: The current findings support that P. africanum induces anxiolytic and analgesic effects in a sodium valproate-induced autistic disorders model.
Keywords: Piptadeniastrum africanum; analgesic; anxiolytic; autistic disorders; sodium valproate.
© 2025 The Author(s). Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures












References
-
- Autism Spectrum Disorders Working Group of The Psychiatric Genomics Consortium . 2017. “Meta‐Analysis of GWAS of Over 16,000 Individuals With Autism Spectrum Disorder Highlights a Novel Locus at 10q24.32 and a Significant Overlap With Schizophrenia.” Molecular Autism 8: 21. 10.1186/s13229-017-0137-9. - DOI - PMC - PubMed
-
- Ay, H. , Horata E., Öncü Kaya E. M., and Korkma O. T.. 2024. “Increased Serotonin Levels and Unchanged Glutamate and GABA Levels in Thalamic Microdialysates Despite Reduced Cell Numbers in a Valproic Acid‐Induced Autism Model.” Neurochemical Research 50, no. 1: 45. 10.1007/s11064-024-04299-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

