Claudin-11 Enhances Invasive and Metastatic Abilities of Small-Cell Lung Cancer Through MT1-MMP Activation
- PMID: 40079504
- PMCID: PMC12127104
- DOI: 10.1111/cas.70038
Claudin-11 Enhances Invasive and Metastatic Abilities of Small-Cell Lung Cancer Through MT1-MMP Activation
Abstract
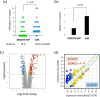
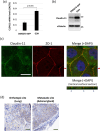
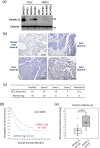
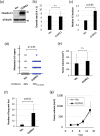
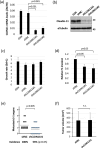
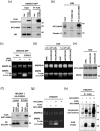
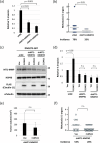
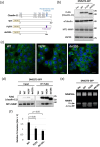
Small-cell lung cancer (SCLC) is an aggressive tumor characterized by the frequent development of distant metastases. This study aimed to explore the mechanism of SCLC metastasis using an originally developed orthotopic transplantation model with DMS273 cells. An analysis of G3H cells, a highly metastatic subline of DMS273 cells, revealed that claudin-11 promotes the invasive and metastatic ability of the cells. Further analysis revealed that membrane type 1-matrix metalloproteinase (MT1-MMP), which degrades a wide range of extracellular matrix components, was coprecipitated with claudin-11. Gelatin zymography revealed that claudin-11 enhanced MT1-MMP activity, and MT1-MMP silencing suppressed the invasive and metastatic ability of G3H cells. Moreover, in MT1-MMP silencing DMS273 cells, the enhancement of invasion and metastatic potential induced by CLDN11 overexpression was abolished. These results demonstrate that claudin-11 enhances the invasive capacity of the cells by activating MT1-MMP, which promotes metastatic formation in the orthotopic transplantation model. Additionally, claudin-11 expression was detected in SCLC tumor samples, and higher expression of CLDN11 correlated with poor prognosis in patients with SCLC. These findings suggest that the claudin-11/MT1-MMP axis plays an important role in SCLC pathogenesis.
Keywords: MT1‐MMP; SCLC; claudin‐11; invasion; metastasis.
© 2025 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Masanori Hatakeyama is the Editor‐in‐Chief of Cancer Science. The others have no conflicts of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

