Silylation of Aryl and Alkyl Chlorides by a Seven-Membered Dialkoxysilyl Group Si(pan)Me via an In Situ Generated Silylpotassium
- PMID: 40079693
- PMCID: PMC12087862
- DOI: 10.1002/anie.202424183
Silylation of Aryl and Alkyl Chlorides by a Seven-Membered Dialkoxysilyl Group Si(pan)Me via an In Situ Generated Silylpotassium
Abstract
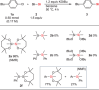
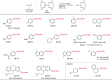
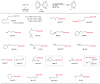
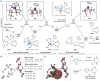
Silicon-containing compounds are increasingly vital in pharmaceutical and agrochemical applications, yet existing silylation methods face critical limitations: poor reactivity of unactivated silanes and instability of activated silylation reagents and their products. Here, we present a seven-membered dialkoxysilyl unit, dioxasilepane, abbreviated as Si(pan), that combines exceptional stability with controllable reactivity. We demonstrate a versatile method for Si(pan)Me incorporation into organic molecules through reactions with diverse aryl, alkenyl, and alkyl chlorides. Notably, we have isolated and structurally characterized the key silylpotassium intermediate as its 18-crown-6 complex through X-ray crystallography. Experimental mechanistic studies reveal that this silylpotassium species mediates the transformation primarily through halogen-metal exchange (HME). Computational investigations confirm the HME pathway while suggesting a concurrent SN2 mechanism for specific primary alkyl chlorides. This methodology establishes Si(pan) as a robust building block for constructing silicon-containing molecular frameworks, addressing a longstanding challenge in organic synthesis.
Keywords: Alkoxysilane; Dioxasilepane; Halogen‐metal exchange; Silylation; Silylpotassium.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Tacke R., Zilch H., Endeavour 1986, 10, 191–197. - PubMed
-
- Pooni P. K., Showell G. A., Mini‐Rev. Med. Chem. 2006, 6, 1169–1177. - PubMed
-
- Meanwell N. A., J. Med. Chem. 2011, 54, 2529–2591. - PubMed
-
- Tacke R., Dörrich S., in Atypical Elements in Drug Design (Ed: Schwartz J.), Springer International Publishing, Cham, 2014, pp. 29–59.
-
- Fujii S., Hashimoto Y., Future Med. Chem. 2017, 9, 485–505. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

