Finely Tuned γ Tracks Medication Cycles in Parkinson's Disease: An Ambulatory Brain-Sense Study
- PMID: 40079832
- PMCID: PMC12089918
- DOI: 10.1002/mds.30160
Finely Tuned γ Tracks Medication Cycles in Parkinson's Disease: An Ambulatory Brain-Sense Study
Abstract
Background: Novel commercial brain-sense neurostimulators enable us to contextualize brain activity with symptom and medication states in real-life ambulatory settings in Parkinson's disease (PD). Although various candidate biomarkers have been proposed for adaptive deep brain stimulation (DBS), a comprehensive comparison of their ambulatory profiles is lacking.
Objectives: To systematically compare the ambulatory neurophysiological dynamics and clinical properties of three candidate biomarkers-low-frequency, beta (β), and finely tuned γ (FTG) activity.
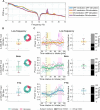
Methods: We investigated 14 PD patients implanted with the Medtronic Percept PC, who underwent up to two 4-week ambulatory multimodal recording periods on their regular medication and stimulation. Subthalamic nucleus local field potentials (LFPs) of low-frequency, β, and FTG activity were recorded. Additionally, objective motor symptom states, physical activity and heart rate using wearables, as well as medication-intake times, sleep-awake times, and subjective symptom states using diaries were co-registered. LFP dynamics were also compared to high-resolution in-hospital recordings under off/on dopaminergic medication and stimulation conditions.
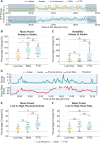
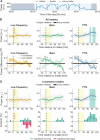
Results: FTG reliably indexed off to on medication states in the ambulatory setting at the group and individual levels, and these spectral dynamics could be anticipated by high-resolution in-hospital recordings. Both FTG and low-frequency correlated with wearable-based dyskinesia scores, whereas diary-based dyskinesia events were only linked to FTG. Importantly, FTG indicated on-medication states regardless of the presence of dyskinesia and despite potential motion and heart rate artifacts. The 24-hour profile revealed large circadian power shifts that may overdrive medication-intake dynamics.
Conclusion: Despite the limitations of low-temporal resolution recordings, this work provides valuable insights into the real-life dynamics of biomarkers. Specifically, it highlights the utility of FTG as a primary and reliable indicator of medication states for adaptive DBS. © 2025 The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: Parkinson's disease; biomarkers; brain‐sense; closed‐loop, adaptive deep brain stimulation; home recording.
© 2025 The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Figures


References
-
- Bronte‐Stewart H, Beudel M, Ostrem JLA, et al. Adaptive DBS algorithm for personalized therapy in Parkinson's disease: ADAPT‐PD clinical trial methodology and early data (P1‐11.002). Neurology 2023;100(17_supplement_2):3204. 10.1212/wnl.0000000000203099 - DOI
MeSH terms
Substances
Grants and funding
- Gottfried und Julia Bangerter-Rhyner-Stiftung
- Department of Neurology, Bern University Hospital
- 32003BL_197709/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- 32003B_205156 / 2/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- PZ00P3_202166/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
LinkOut - more resources
Full Text Sources
Medical

