The cerebellum in epilepsy
- PMID: 40079849
- PMCID: PMC12169398
- DOI: 10.1111/epi.18316
The cerebellum in epilepsy
Abstract
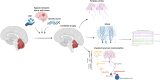
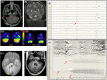
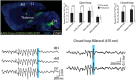
The cerebellum, a subcortical structure, is traditionally linked to sensorimotor integration and coordination, although its role in cognition and affective behavior, as well as epilepsy, is increasingly recognized. Cerebellar dysfunction in patients with epilepsy can result from genetic disorders, antiseizure medications, seizures, and seizure-related trauma. Impaired cerebellar function, regardless of cause, can cause ataxia (imbalance, impaired coordination, unsteady gait), tremor, gaze-evoked nystagmus, impaired slow gaze pursuit and saccade accuracy, as well as speech deficits (slurred, scanning, or staccato). We explore how cerebellar dysfunction can contribute to epilepsy, reviewing data on genetic, infectious, and neuroinflammatory disorders. Evidence of cerebellar dysfunction in epilepsy comes from animal studies as well as human neuropathology and structural magnetic resonance imaging (MRI), functional and diffusion tensor MRI, positron emission and single photon emission computerized tomography, and depth electrode electro-encephalography studies. Cerebellar lesions can infrequently cause epilepsy, with focal motor, autonomic, and focal to bilateral tonic-clonic seizures. Antiseizure medication-resistant epilepsy typically presents in infancy or before age 1 year with hemifacial clonic or tonic seizures ipsilateral to the cerebellar mass. Lesions are typically asymmetric benign or low-grade tumors in the floor of the fourth ventricle involving the cerebellar peduncles and extending to the cerebellar hemisphere. Electrical stimulation of the cerebellum has yielded conflicting results on efficacy, although methodological issues confound interpretation. Epilepsy-related comorbidities including cognitive and affective disorders, falls, and sudden unexpected death in epilepsy may also be impacted by cerebellar dysfunction. We discuss how cerebellar dysfunction may drive seizures and how genetic epilepsies, seizures and seizure therapies may drive cerebellar dysfunction, and how our understanding of epilepsy-related comorbidities through basic neuroscience, animals models and patient studies can advance our understanding and improve patient outcomes.
Keywords: cerebellum; epilepsy; neuropathology; stimulation.
© 2025 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
Christopher Elder is a paid speaker for SK Life Sciences, Inc. Rebecca Kerestes has no conflict of interest. Maria Marchese has no conflict of interest. Puneet Opal has served as site principal investigator for a clinical trial sponsored by Biohaven Pharmaceuticals.
Figures
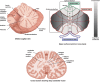

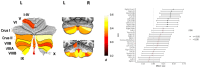
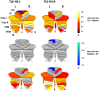
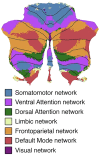
References
-
- Barton RA, Venditti C. Rapid evolution of the cerebellum in humans and other great apes. Curr Biol. 2014;24:2440–2444. - PubMed
-
- D'Angelo E. Physiology of the cerebellum. Handbook Clin Neuro. 2018;154:85–108. - PubMed
-
- Schmahmann JD. The cerebellum and cognition. Neurosci Lett. 2019;688:62–75. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

