Adjuvant PD-1 Blockade With Camrelizumab for Nasopharyngeal Carcinoma: The DIPPER Randomized Clinical Trial
- PMID: 40079940
- PMCID: PMC11907361
- DOI: 10.1001/jama.2025.1132
Adjuvant PD-1 Blockade With Camrelizumab for Nasopharyngeal Carcinoma: The DIPPER Randomized Clinical Trial
Abstract
Importance: Approximately 20% to 30% of patients with locoregionally advanced nasopharyngeal carcinoma (NPC) experience disease relapse despite definitive chemoradiotherapy. The programmed cell death 1 (PD-1) blockade camrelizumab has demonstrated considerable value in recurrent or metastatic NPC, while its role in locoregionally advanced NPC is unclear.
Objective: To evaluate the efficacy and safety of adjuvant camrelizumab for patients with locoregionally advanced NPC.
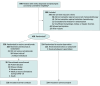
Design, setting, and participants: Randomized, open-label, multicenter, phase 3 clinical trial conducted from August 2018 to November 2021 at 11 centers in China and enrolling 450 patients with T4N1M0 or T1-4N2-3M0 NPC who had completed induction-concurrent chemoradiotherapy. The final date of follow-up was March 20, 2024.
Interventions: Patients were randomized (1:1) to receive adjuvant camrelizumab (200 mg intravenously once every 3 weeks for 12 cycles; n = 226) or observation (standard therapy group; n = 224).
Main outcomes and measures: The primary end point was event-free survival (freedom from distant metastasis, locoregional relapse, or death due to any cause). Secondary end points included distant metastasis-free survival, locoregional relapse-free survival, overall survival, safety, and health-related quality of life.
Results: Among the 450 participants (mean age, 45 [SD, 10] years; 24% women), after a median follow-up of 39 (IQR, 33-50) months, the camrelizumab group had a 3-year event-free survival rate of 86.9%, whereas the standard therapy group had a rate of 77.3% (stratified hazard ratio, 0.56; 95% CI, 0.36-0.89; P = .01). Grade 3 or 4 adverse events were reported in 23 patients (11.2%) in the camrelizumab and 7 (3.2%) in the standard therapy group. Reactive capillary endothelial proliferation was the most common adverse event related to camrelizumab, occurring in 85.8% of patients at grade 1 or 2, while 2% of patients had grade 3 or 4 events. There was no significant deterioration in quality of life associated with camrelizumab treatment.
Conclusions and relevance: Adjuvant PD-1 blockade with camrelizumab significantly improved event-free survival with manageable toxicities, highlighting its potential role in the management of locoregionally advanced NPC.
Trial registration: ClinicalTrials.gov Identifier: NCT03427827.
Conflict of interest statement
Figures

Comment in
-
Curative Application of Immunotherapy in Untreated Head and Neck and Nasopharynx Cancer.JAMA. 2025 May 13;333(18):1584-1585. doi: 10.1001/jama.2025.3012. JAMA. 2025. PMID: 40080388 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

