Tape strip expression profiling of juvenile dermatomyositis skin reveals mitochondrial dysfunction contributing to disease endotype
- PMID: 40080076
- PMCID: PMC12016934
- DOI: 10.1172/jci.insight.179875
Tape strip expression profiling of juvenile dermatomyositis skin reveals mitochondrial dysfunction contributing to disease endotype
Abstract
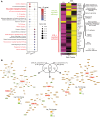
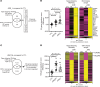
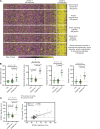
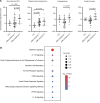
Skin inflammation in juvenile dermatomyositis (JDM) can signal disease onset or flare, and the persistence of cutaneous disease can prevent complete disease remission. The noninvasive study of cutaneous expression signatures through tape stripping (TS) holds the potential to reveal mechanisms underlying disease heterogeneity and organ-specific inflammation. The objectives of this study were to (a) define TS expression signatures in lesional and nonlesional JDM skin, (b) analyze TS signatures to identify JDM disease endotypes, and (c) compare TS and blood signatures. Although JDM lesional skin demonstrated interferon signaling as the top upregulated pathway, nonlesional skin uniquely highlighted pathways involved in metabolism, angiogenesis, and calcium signaling. Both lesional and nonlesional skin shared inflammasome pathway dysregulation. Using unsupervised clustering of skin expression data, we identified a treatment-refractory JDM subgroup distinguished by upregulation of genes associated with mitochondrial dysfunction. The treatment-refractory JDM subgroup also demonstrated higher interferon, angiogenesis, and innate immune expression scores in skin and blood, though scores were more pronounced in skin as compared with blood. TS expression signatures in JDM provided insight into disease mechanisms and molecular subgroups. Skin, as compared with blood, transcriptional profiles served as more sensitive markers to classify disease subgroups and identify candidate treatment targets.
Keywords: Autoimmune diseases; Autoimmunity; Clinical practice; Dermatology; Expression profiling; Inflammation.
Figures

References
-
- Mathiesen P, et al. Long-term outcome in patients with juvenile dermatomyositis: a cross-sectional follow-up study. Scand J Rheumatol. 2012;41(1):50–58. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

