The role of the intestinal microbiome in cognitive decline in patients with kidney disease
- PMID: 40080091
- PMCID: PMC11905753
- DOI: 10.1093/ndt/gfae253
The role of the intestinal microbiome in cognitive decline in patients with kidney disease
Abstract
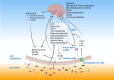
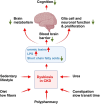
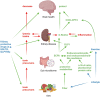
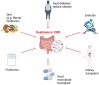
Cognitive decline is frequently seen in patients with chronic kidney disease (CKD). The causes of cognitive decline in these patients are likely to be multifactorial, including vascular disease, uraemic toxins, blood-brain barrier leakage, and metabolic and endocrine changes. Gut dysbiosis is common in patients with CKD and contributes to the increase in uraemic toxins. However, the gut microbiome modulates local and systemic levels of several metabolites such as short-chain fatty acids or derivatives of tryptophan metabolism, neurotransmitters, endocannabinoid-like mediators, bile acids, hormones such as glucagon-like peptide 1 (GLP1) or cholecystokinin (CCK). These factors can affect gut function, immunity, autonomic nervous system activity and various aspects of brain function. Key areas include blood-brain barrier integrity, nerve myelination and survival/proliferation, appetite, metabolism and thermoregulation, mood, anxiety and depression, stress and local inflammation. Alterations in the composition of the gut microbiota and the production of biologically active metabolites in patients with CKD are well documented and are favoured by low-fiber diets, elevated urea levels, sedentary lifestyles, slow stool transit times and polypharmacy. In turn, dysbiosis can modulate brain function and cognitive processes, as discussed in this review. Thus, the gut microbiome may contribute to alterations in cognition in patients with CKD and may be a target for therapeutic interventions using diet, prebiotics and probiotics.
Keywords: chronic kidney disease; cognition; exerkines; gut microbiome; short chain fatty acids.
© The Author(s) 2025. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
C.A.W. reports honoraria from Kyowa Kirin and Medice, and collaborations with Chugai and Bayer AG. A.O. has received grants from Sanofi and consultancy or speaker fees or travel support from Adviccene, Alexion, Astellas, AstraZeneca, Amicus, Amgen, Bioporto, Boehringer Ingelheim, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Sobi, Menarini, Mundipharma, Kyowa Kirin, Lilly, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex and Vifor Fresenius Medical Care Renal Pharma and Spafarma, and is Director of the Catedra UAM-Astrazeneca of chronic kidney disease and electrolytes. He has stock in Telara Farma. R.U. is Professor Emeritus UCL and is currently employed part-time as a Chief Scientist (kidney diseases) by AstraZeneca Biopharmaceuticals R&D, Cambridge, UK. L.G. received grant support from Abionyx and Sanofi to his University department (DIMEPRE-); he has been a member of advisory boards for AstraZeneca, Baxter, Chinook, GSK, Mundipharma, Novartis, Pharmadoc, Roche, Sanofi, Travere and Vifor Pharma, and an invited speaker at meetings supported by AstraZeneca, Astellas, Estor, Fresenius, Werfen, Medtronic, Travere and GSK. Z.A.M. reports grants from NATIONAL RESEARCH AGENCY, during the conduct of the study; grants from Amgen, grants from Sanofi-Genzyme, grants from French Government, grants from MSD, grants and other from GSK, grants from Lilly, grants from Fresenius Medical Care, grants from Baxter, grants from Otsuka, grants and other from AstraZeneca, grants from Vifor and other from Boehringer, outside the submitted work.
Figures

References
-
- Viggiano D. Mild cognitive impairment and kidney disease: clinical aspects. Nephrol Dial Transplant 2020;35:10–17. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

