Compassionate Access to Brigatinib for Patients with Non-small-cell Lung Cancer Harboring a ROS1 Rearrangement: Results from the BRIGAROS Study
- PMID: 40080277
- PMCID: PMC11933156
- DOI: 10.1007/s11523-025-01131-x
Compassionate Access to Brigatinib for Patients with Non-small-cell Lung Cancer Harboring a ROS1 Rearrangement: Results from the BRIGAROS Study
Abstract
Background: ROS1 chromosomic rearrangement is a rare oncogenic driver, and patients with this rearrangement benefit from specific targeted treatments in the first-line setting. However, therapeutic options are limited in pretreated patients. Brigatinib is a validated drug for ALK rearrangements, and also has an in vitro activity against ROS1. In vivo efficacy is also suggested in some clinical series.
Objective: We aimed to specifically study brigatinib in patients with pretreated advanced non-small-cell lung cancer (NSCLC).
Methods: We retrospectively collected data from 20 centers in France. Brigatinib was delivered through a compassionate use program in France between 2018 and 2020. The primary endpoint was progression-free survival. Secondary endpoints were the objective response rate, overall survival, and tolerance.
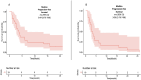
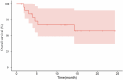
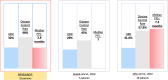
Results: Twenty-five patients treated with brigatinib were included in our study. All patients were pretreated, and all of them previously received crizotinib. Median progression-free survival was 3.8 months (95% confidence interval 2.8-7.1). The objective response rate was 32%, with a disease control rate of 48%. Three patients had a prolonged response of more than 18 months at the end of data collection. We did not identify factors predictive of prolonged response. There were no grade 4 or 5 toxicities.
Conclusion: Brigatinib may represent an interesting therapeutic option for patients who have progressed after standard treatments.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Author contribution: Jean Mourlanette, Julien Mazieres, and Siham Mallah wrote this article, centered the data in Toulouse, and made statistical analyses. Gaelle Rousseau-Bussac and Christos Chouaid promoted the study, and helped retrieving data. All co authors participated in data collection. Funding: No external funding was used in the preparation of this article. Conflict of interest: All authors were asked to make their statement of potential conflicts of interest on the site https://coi.asco.org/ . Jean Mourlanette, Gaelle Rousseau-Bussac, Siham Mallah, Florian Guisier, Gerard Zalcman, Rémi Veillon, Clarisse Audigier-Valette, Magali Roa, Isabelle Nicolle, Helene Doubre, Nicolas Cloarec, Régine Lamy, Hugues Morel, Hubert Curcio, Aurélie Lagrange, Roland Schott, Marielle Sabatini, Anne Claire Toffart, Julian Pinsolle, Jaafar Bennouna, Christos Chouaid, and Julien Mazieres declare that they have no conflicts of interest that might be relevant to the contents of this article. Ethics Approval: The ethics committee of the Société de Pneumologie de Langue Française approved the study (number: CEPRO 2021-002). Consent to Participate: Not applicable, a letter of information was given to patients alive. Consent to Publish: Not applicable. Availability of Data and Materials: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. Code Availability: Not applicable.
Figures
References
-
- Ou S-HI, Tan J, Yen Y, Soo RA. ROS1 as a ‘druggable’ receptor tyrosine kinase: lessons learned from inhibiting the ALK pathway. Expert Rev Anticancer Ther. 2012;12(4):447–56. - PubMed
-
- Stumpfova M, Jänne PA. Zeroing in on ROS1 Rearrangements in Non-Small Cell Lung Cancer. Clin Cancer Res. 2012;18(16):4222–4. - PubMed
-
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the college of american pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn. 2018;20(2):129–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

