Compressive Forces Induce Epigenetic Activation of Aged Human Dermal Fibroblasts Through ERK Signaling Pathway
- PMID: 40080399
- PMCID: PMC12151880
- DOI: 10.1111/acel.70035
Compressive Forces Induce Epigenetic Activation of Aged Human Dermal Fibroblasts Through ERK Signaling Pathway
Abstract
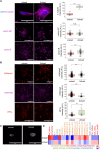
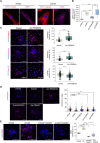
Age-related changes in human dermal fibroblasts (HDFs) contribute to impaired wound healing and skin aging. While these changes result in altered mechanotransduction, the epigenetic basis of rejuvenating aging cells remains a significant challenge. This study investigates the effects of compressive forces on nuclear mechanotransduction and epigenetic rejuvenation in aged HDFs. Using a compressive force application model, the activation of HDFs through alpha-smooth muscle actin (ɑ-SMA) is demonstrated. Sustained compressive forces induce significant epigenetic modifications, including chromatin remodeling and altered histone methylation patterns. These epigenetic changes correlate with enhanced cellular migration and rejuvenation. Small-scale drug screening identifies the extracellular signal-regulated kinase (ERK) signaling pathway as a key mediator of compression-induced epigenetic activation. Furthermore, implanting aged cell spheroids into an aged skin model and subjecting the tissue to compressive forces resulted in increased collagen I protein levels. Collectively, these findings demonstrate that applying compressive force to aged fibroblasts activates global epigenetic changes through the ERK signaling pathway, ultimately rejuvenating cellular functions with potential applications for wound healing and skin tissue regeneration.
Keywords: ERK signaling pathway; epigenetic activation; human dermal fibroblasts; mechanical rejuvenation.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

