YAP1 is a key regulator of EWS::FLI1-dependent malignant transformation upon IGF-1-mediated reprogramming of bone mesenchymal stem cells
- PMID: 40080499
- PMCID: PMC11936874
- DOI: 10.1016/j.celrep.2025.115381
YAP1 is a key regulator of EWS::FLI1-dependent malignant transformation upon IGF-1-mediated reprogramming of bone mesenchymal stem cells
Abstract
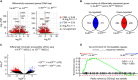
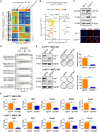
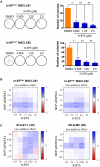
Ewing sarcoma (EwS) is an aggressive cancer of adolescents in need of effective treatment. Insulin-like growth factor (IGF)-1 is an autocrine growth factor for EwS, but only 10% of patients respond to IGF-1 receptor (IGF-1R) blockade. Although EwS is presumed to originate from mesenchymal progenitors during bone development, targeting of the EwS driver oncogene EWS::FLI1 to the mesenchymal lineage in a mouse model does not result in tumor formation but in skeletal malformations and perinatal death. We report that transient exposure to IGF-1 concentrations mimicking serum levels during puberty reprograms limb-derived mesenchymal cells of EWS::FLI1-mutant mice to stable transformation and tumorigenicity. We identify a modular mechanism of IGF-1-driven tumor promotion in the early steps of EwS pathogenesis, in which Yap1 plays a central role. Pharmacologic Yap1/Tead inhibition reverses the transformed phenotype of EWS::FLI1-expressing cells. Our data provide a rationale for combined IGF-1R and YAP/TEAD inhibition in the treatment of EwS patients.
Keywords: CP: Stem cell research; EWS::FLI1; Ewing sarcoma; IGF-1/insulin signaling; YAP1/TAZ signaling; endochondral bone development; mesenchymal stem cells; puberty.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Davis L., Malempati S. Ewing sarcoma in adolescents and young adults: diagnosis and treatment. Clin. Oncol. Adolesc. Young Adults. 2014;4:21–31. doi: 10.2147/COAYA.S61451. - DOI
-
- Minas T.Z., Surdez D., Javaheri T., Tanaka M., Howarth M., Kang H.J., Han J., Han Z.Y., Sax B., Kream B.E., et al. Combined experience of six independent laboratories attempting to create an Ewing sarcoma mouse model. Oncotarget. 2017;8:34141–34163. doi: 10.18632/oncotarget.9388. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

