Efficacy and Safety of Higher Doses of Levofloxacin for Multidrug-resistant Tuberculosis: A Randomized, Placebo-controlled Phase II Clinical Trial
- PMID: 40080768
- PMCID: PMC12213226
- DOI: 10.1164/rccm.202407-1354OC
Efficacy and Safety of Higher Doses of Levofloxacin for Multidrug-resistant Tuberculosis: A Randomized, Placebo-controlled Phase II Clinical Trial
Abstract
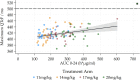
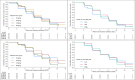
Rationale: Evaluation of optimal dosing has generally been inadequate during tuberculosis (TB) drug development. Fluoroquinolones are central to TB treatment. Objective: To determine the dose of levofloxacin needed to achieve maximal efficacy and acceptable safety and tolerability as part of a multidrug TB regimen. Methods: Opti-Q was an international, multicenter, randomized, placebo-controlled phase II trial. Eligible participants with TB resistant to isoniazid and rifampicin but susceptible to fluoroquinolones were randomized to receive one of four weight-adjusted once-daily doses of levofloxacin for 24 weeks (168 doses) alongside a multidrug regimen: 11 mg/kg (750 mg), 14 mg/kg (750 mg/1,000 mg), 17 mg/kg (1,000 mg/1,250 mg) or 20 mg/kg (1,250 mg/1,500 mg). The primary efficacy outcome was time to sputum culture conversion, and the primary safety outcome was grade ≥3 adverse events (AEs). Measurements and Main Results: A total of 111 participants were randomized from three sites in South Africa and Peru. Eighty-three (75%) had cavities on chest X-ray, 55 (50%) had a smear grading of 3+, and the median body mass index was 20.4 kg/m2. Median levofloxacin areas under the curve (AUCs)/minimum inhibitory concentrations were 573, 633, 918, and 1,343 across the four treatment arms. There was no difference in time to culture conversion on solid or liquid media by treatment arm (stratified log-rank P = 0.282), by tertile of AUC/minimum inhibitory concentration (P = 0.350), or by dose received (P = 0.723); 69.3%, 74.8%, 70.6%, and 78.3% exhibited culture conversion after 8 weeks on solid media, respectively, across the treatment arms; along with 64.6%, 69.5%, 52.6%, and 69.6% on liquid culture. More participants experienced a grade 3-5 AE at higher doses (37.0% and 16.0% in the highest and lowest dose groups, respectively; P = 0.042, Cochran-Armitage test for trend) and higher tertiles of AUC (P = 0.011). Conclusions: As part of a multidrug regimen, doses of levofloxacin >1,000 mg/d resulted in greater exposures and increased frequency of AEs but did not result in faster time to sputum culture conversion. A dose of 1,000 mg/d can achieve the target exposure in nearly all adults and was well tolerated. Clinical trial registered with www.clinicaltrials.gov (NCT01918397).
Keywords: MDR-TB; TB; double-blind randomized controlled trial; levofloxacin.
Figures

Comment in
-
From Repurposing to Refinement: Optimizing Levofloxacin for Treatment of Multidrug-Resistant Tuberculosis.Am J Respir Crit Care Med. 2025 Jul;211(7):1126-1127. doi: 10.1164/rccm.202503-0550ED. Am J Respir Crit Care Med. 2025. PMID: 40315145 Free PMC article. No abstract available.
References
-
- World Health Organization. Geneva: World Health Organization; 2022. WHO consolidated guidelines on drug-sensitive tuberculosis treatment.https://iris.who.int/handle/10665/353829
-
- World Health Organization. Geneva: World Health Organization; 2022. WHO consolidated guidelines on drug-resistant tuberculosis treatment.https://iris.who.int/handle/10665/365308 - PubMed
-
- Nyang’Wa BT, Berry C, Kazounis E, Motta I, Parpieva N, Tigay Z, et al. Short oral regimens for pulmonary rifampicin-resistant tuberculosis (TB-PRACTECAL): an open-label, randomised, controlled, phase 2B-3, multi-arm, multicentre, non-inferiority trial. Lancet Respir Med . 2024;12:117–128. - PubMed
-
- van Ingen J, Aarnoutse RE, Donald PR, Diacon AH, Dawson R, Plemper van Balen G, et al. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis . 2011;52:e194–e199. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

