Positioning Imatinib for Pulmonary Arterial Hypertension: A Dose-Finding Phase 2 Study
- PMID: 40080796
- PMCID: PMC12175952
- DOI: 10.1164/rccm.202410-1929OC
Positioning Imatinib for Pulmonary Arterial Hypertension: A Dose-Finding Phase 2 Study
Abstract
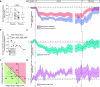
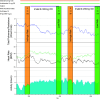
Rationale: Imatinib, 400 mg daily, reduces pulmonary vascular resistance and improves exercise capacity in patients with pulmonary arterial hypertension. Concerns about safety and tolerability limit its use. Objectives: We sought to identify a safe and tolerated dose of oral imatinib between 100 mg and 400 mg daily and evaluate its efficacy. Methods: Oral imatinib was added to the background therapy of 17 patients with pulmonary arterial hypertension, including 13 who were implanted with devices that provide daily measurements of cardiopulmonary hemodynamics and physical activity. The first patient was started on 100 mg daily. The next 12 patients, recruited serially, were started on 200 mg, 300 mg, or 400 mg daily, following a continuous reassessment dose-finding model. An extension cohort (Patients 14-17) received 100 mg or 200 mg daily. Measurements and Main Results: The continuous reassessment model recommended starting dose was 200 mg daily. The most common side effect was nausea. Imatinib reduced mean pulmonary artery pressure (-6.5 mm Hg; 95% confidence interval [CI] = -2.4 to -10.6; P < 0.01) and total pulmonary resistance (-2.8 Wood units; 95% CI = -1.5 to -4.2; P < 0.001), with no significant change in cardiac output. The reduction in total pulmonary resistance was dose and exposure dependent; the reduction from baseline with imatinib, at 200 mg daily, was -20.3% (95% CI = -14.3 to -26.3%). Total pulmonary resistance and night heart rate declined steadily over the first 28 days of treatment and remained below baseline up to 40 days after imatinib withdrawal. Conclusions: Oral imatinib, 200 mg daily, is well tolerated as an add-on treatment for pulmonary arterial hypertension. A delay in the return of cardiopulmonary hemodynamics to baseline was observed after imatinib was stopped.
Keywords: adaptive trial design; implanted hemodynamic sensors; remote monitoring.
Figures

Comment in
-
Another Act in the Story of Oral Imatinib for Pulmonary Arterial Hypertension.Am J Respir Crit Care Med. 2025 Jun;211(6):904-905. doi: 10.1164/rccm.202503-0603ED. Am J Respir Crit Care Med. 2025. PMID: 40315143 Free PMC article. No abstract available.
References
-
- Hassoun PM. Pulmonary arterial hypertension. N Engl J Med . 2021;385:2361–2376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

