O-GlcNAc-modified HOXA9 suppresses ferroptosis via promoting UBR5-mediated SIRT6 degradation in nasopharyngeal carcinoma
- PMID: 40081214
- PMCID: PMC11932873
- DOI: 10.1016/j.neo.2025.101142
O-GlcNAc-modified HOXA9 suppresses ferroptosis via promoting UBR5-mediated SIRT6 degradation in nasopharyngeal carcinoma
Abstract
Background: Nasopharyngeal carcinoma (NPC) is the most common malignancy of the nasopharynx. Ferroptosis induction shows anti-tumor activities in cancers including NPC. Elucidating the regulatory mechanism of ferroptosis is crucial for developing targeted therapeutic strategies for NPC.
Methods: The GEO dataset (GSE68799) was used to analyze HOXA9 expression in NPC. Cell viability, levels of MDA, total iron, Fe2+ and GSH, and lipid peroxidation were examined for ferroptosis evaluation. O-GlcNAcylation levels on HOXA9 and ubiquitination levels on SIRT6 were detected by immunoprecipitation. ChIP and luciferase assays were applied for determining the interaction of HOXA9 and UBR5. The interaction between UBR5 and SIRT6, OGT and HOXA9 were evaluated by Co-IP assays. A subcutaneous NPC mouse model was established to explore whether knockdown of HOXA9 or UBR5 regulates tumor growth in vivo.
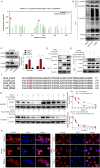
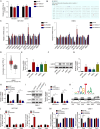
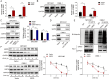
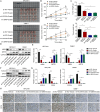
Results: HOXA9 was highly expressed in NPC, and knockdown of HOXA9 elevated total iron, Fe2+ and lipid peroxidation and reduced GSH and NPC cell viability. O-GlcNAcylation stabilized HOXA9 and facilitated its nuclear translocation in NPC cells. HOXA9 directly bound to UBR5 promoter to increase its expression, thus accelerating ubiquitination and degradation of SIRT6. HOXA9 restrained ferroptosis via promoting UBR5 expression, and UBR5 suppressed ferroptosis through promotion of SIRT6 ubiquitination and degradation. Knockdown of HOXA9 or UBR5 promoted ferroptosis and inhibited NPC growth in mice.
Conclusion: O-GlcNAc-modified HOXA9 inhibits ferroptosis by enhancing UBR5 expression and ubiquitination and degradation of SIRT6 in NPC cells, thus accelerating NPC progression. Our study provides potential therapeutic targets for NPC treatment.
Keywords: Ferroptosis; HOXA9; Nasopharyngeal carcinoma; O-GlcNAcylation; SIRT6; UBR5.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Lee A.W., Ma B.B., Ng W.T., Chan A.T. Management of nasopharyngeal carcinoma: current practice and future perspective. J. Clin. Oncol.: Official J. Am. Society Clin. Oncol. 2015;33(29):3356–3364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

