Real-world treatment patterns and outcomes based on RAS/BRAF status in metastatic colorectal cancer-Analysis of the Prospective Dutch Colorectal Cancer cohort
- PMID: 40081858
- PMCID: PMC12141976
- DOI: 10.1002/ijc.35410
Real-world treatment patterns and outcomes based on RAS/BRAF status in metastatic colorectal cancer-Analysis of the Prospective Dutch Colorectal Cancer cohort
Abstract
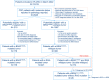
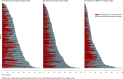
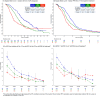
The treatment landscape for metastatic colorectal cancer (mCRC) has evolved into a continuum of care with an essential role for biomarkers and molecular subgroups. Treatment guidelines are primarily based on trial results; however, populations and outcomes differ from clinical practice. To support the interpretation of trial results and to assist in tailored patient counseling, we evaluated real-world treatment patterns and outcomes according to RAS/BRAF status. We included all patients diagnosed with BRAFV600E-mutated mCRC in 2015-2020, participating in the Prospective Dutch Colorectal Cancer cohort study, plus a 1:2 random selection of patients with RAS-mutated and double wild-type mCRC. We evaluated differences in administered lines of treatment (LOTs), local treatment, attrition rates, treatment duration, progression-free survival (PFS) and overall survival (OS). 178 BRAFV600E-mutated, 221 RAS-mutated, and 174 double wild-type patients were included. Of BRAFV600E-mutated patients, 26% received ≥3 LOTs, compared to 42% and 47% of the RAS-mutated and double wild-type patients, respectively (p = .002). Local treatment was performed in 25% of BRAFV600E-mutated, 43% of RAS-mutated, and 49% of double wild-type patients (p < .001). Median OS from diagnosis was 15.4, 24.1, and 32.6 months, respectively (p < .001) and loss of prognostic value of RAS/BRAF was observed from the 3rd LOT onwards (p = .17 and p = .54). This paper provides a comprehensive overview of the treatment landscape of mCRC per RAS/BRAF status in daily clinical practice. The observed substantial treatment heterogeneity within and between molecular subgroups underlines the importance of collecting real-world data to address post-trial knowledge gaps and to optimize individualized counseling for all mCRC patients.
Keywords: BRAF; RAS; metastatic colorectal cancer; real‐world; treatment patterns.
© 2025 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
Authors SN, KZ, ME, and PS declare no conflicts of interest. AM reports receiving institutional funds for the development of e‐learning on cancer and exercise for Eli Lilly. GV reports receiving institutional grants from BMS, Merck, Servier, Personal Genome Diagnostics, Bayer, Sirtex, Natera, Nordic Pharma, Pierre Fabre, and Delfi Diagnostics. FB declares receiving institutional grants from Personal Genome Diagnostics (PGDx). JR reports receiving institutional grants from BMS, Merck, Delphi, HUB4 Organoids, Cleara, Pierre Fabre, Servier, Xilis, GSK, Incyte, DoMore diagnostics, and PGDx. JR also reports taking part in the advisory board of Merck‐Serono, Pierre Fabre, Servier, BMS, Roche, Bayer, GSK, and Incyte, as well as being a member of the board of directors of the Foundation Hubrecht Organoid Biobank. JR declares that all financial compensation for these roles was institutional. MK reports receiving institutional scientific grants or payments for an advisory role from Amgen, Bayer, Bristol Myers Squibb, GSK, Merck‐Serono, Nordic Pharma, Personal Genome Diagnostics (PGDx), Pierre Fabre, Roche, Servier, Sirtex, and Sanofi‐Aventis. In addition, she reports the following non‐financial interests: PI PLCRC (national observational cohort study), chair of the ESMO RWD & digital health working group, and involvement in several clinical trials as PI or co‐investigator in colorectal cancer.
Figures


References
-
- Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325:669‐685. http://www.ncbi.nlm.nih.gov/pubmed/33591350 - PubMed
-
- McLean J, Rho YS, Kuruba G, et al. Clinical practice patterns in chemotherapeutic treatment regimens for metastatic colorectal cancer. Clin Colorectal Cancer. 2016;15:135‐140. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

