Leveraging T cell co-stimulation for enhanced therapeutic efficacy of trispecific antibodies targeting prostate cancer
- PMID: 40081942
- PMCID: PMC11907039
- DOI: 10.1136/jitc-2024-010140
Leveraging T cell co-stimulation for enhanced therapeutic efficacy of trispecific antibodies targeting prostate cancer
Erratum in
-
Correction: Leveraging T cell co-stimulation for enhanced therapeutic efficacy of trispecific antibodies targeting prostate cancer.J Immunother Cancer. 2025 Mar 26;13(3):e010140corr1. doi: 10.1136/jitc-2024-010140corr1. J Immunother Cancer. 2025. PMID: 40139838 Free PMC article. No abstract available.
Abstract
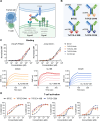
Background: Clinical trials have demonstrated the efficacy of bispecific antibodies in eliciting potent antitumor responses by redirecting T cells to target cancer cells, particularly for the treatment of hematologic malignancies. However, their efficacy against solid tumors is limited by intratumoral T-cell dysfunction and inadequate persistence. The co-stimulatory domains of 4-1BB, OX40, and CD28 are most widely used in engineering chimeric antigen receptor T-cells to augment T-cell responses.
Methods: In this study, we designed three co-stimulatory trispecific T cell-engaging antibodies (TriTCEs) that target Prostate-specific membrane antigen, CD3, and an additional co-stimulatory receptor(OX40, 4-1BB, or CD28). We conducted comparative profiling of the attributes of distinct co-stimulatory signals to T-cell functions in prostate cancer models.
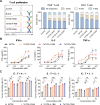
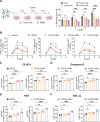
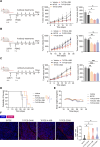
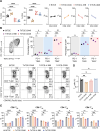
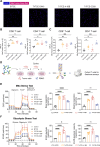
Results: Co-stimulatory trispecific T-cell engagers enhance T-cell activation, proliferation, and display tumor cell-killing activity in vitro. These trispecific antibodies further boosted antitumor activity in humanized mouse xenograft models and increased the infiltration of CD45+ immune cells into solid tumors. Specifically, TriTCE-4-1BB and TriTCE-CD28 selectively promoted the expansion of effector memory T cells and increased the presence of CD4+ T cells more than TriTCE-OX40. T cells stimulated with TriTCE-4-1BB exhibited reduced exhaustion. Furthermore, T cells treated with co-stimulatory trispecific antibodies demonstrated enhanced metabolic activity characterized by increased oxidative phosphorylation and elevated glycolysis.
Conclusions: Collectively, incorporating co-stimulatory receptor targeting domains represents a potentially effective strategy to unlock the full therapeutic potential of T-cell-engaging antibodies for the treatment of solid tumors.
Keywords: Antibody; Immunotherapy; co-stimulatory molecules.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
