Targeting the retinoid signaling pathway with YCT-529 for effective and reversible oral contraception in mice and primates
- PMID: 40082579
- PMCID: PMC11906769
- DOI: 10.1038/s43856-025-00752-7
Targeting the retinoid signaling pathway with YCT-529 for effective and reversible oral contraception in mice and primates
Abstract
Background: The retinoic acid receptor alpha (Rarα) has been validated as a male contraceptive target by genetic knockouts resulting in male sterility. The effects on spermatogenesis in the absence of RARα resemble the loss of RAR signaling in vitamin A deficiency, and the mice are otherwise normal. The effects on spermatogenesis in animals treated orally with the dual RARα/RARγ antagonist BMS-189453 closely phenocopies the absence of RARα function. Notably, the resulting male sterility is reversible. We, therefore, wished to identify RARα-selective inhibitors for potential male non-hormonal contraception.
Methods: YCT-529 was investigated for RARα selective inhibition, physicochemical characteristics, oral bioavailability, and pharmacokinetic properties in mice and non-human primates. It was assessed in mouse mating trials to determine the most effective dosing regimen to induce infertility in male mice and in male non-human primates to reduce sperm levels.
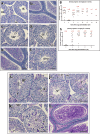
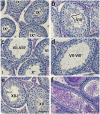
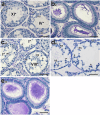
Results: Characterization of YCT-529 shows suitable biochemical, physicochemical, and pharmacokinetic properties for in vivo testing. YCT-529 inhibits mouse fertility of male mice within 4 weeks of oral administration, correlating with disrupted spermatogenesis demonstrating specific inhibition of the RARα pathway. Within 6 weeks after cessation of dosing, mouse fertility reverses. Furthermore, YCT-529 inhibits sperm production in a non-human primate model within 2 weeks of oral dosing without adverse side effects. Within 10-15 weeks after cessation of dosing, non-human primates' sperm counts fully reverses.
Conclusions: These results lay the groundwork for evaluating YCT-529 in human clinical trials.
Plain language summary
There is currently no oral birth control option available to men to prevent pregnancy in their sexual partners. YCT-529 is a non-hormonal male contraceptive that could be a potential drug for men to take to prevent pregnancy. YCT-529 works by interfering with vitamin A signaling necessary for sperm production and fertility. Our study examined its effectiveness and side effects in mice and non-human primates. We show that this oral drug causes infertility in mice, which can be reversed after stopping its consumption. In male non-human primates, sperm production is inhibited (this is how the drug prevents pregnancy) within 2 weeks of starting YCT-529 without adverse side effects and the animals regain fertility after stopping treatment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: N.M. is co-founder and CSO of YourChoice Therapeutics; A.B. is co-founder and CEO of YourChoice Therapeutics; The Regents of the University of Minnesota hold a patent related to this work (Publication No.US-2022-0388993-A 1, Publication Date: 12/08/2022); G.I.G. and N.C. are listed as inventors; YourChoice Therapeutics holds the exclusive license for the IP owned by the University of Minnesota; G.I.G. is a consultant with YourChoice Therapeutics.
Figures



References
-
- Wang, C. & Swerdloff, R. S. Approaches to contraceptive methods for men. (Oxford University Press, 2022).
Grants and funding
- HHSN275201300017C/HD/NICHD NIH HHS/United States
- 5P50HD093540/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- 5P50HD093540/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
LinkOut - more resources
Full Text Sources

