Immune training enhances anti-viral responses and improves outcomes in Pax5-/+ mice susceptible to chronic infection
- PMID: 40082582
- PMCID: PMC11982562
- DOI: 10.1038/s44321-025-00208-4
Immune training enhances anti-viral responses and improves outcomes in Pax5-/+ mice susceptible to chronic infection
Abstract
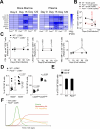
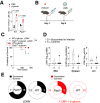
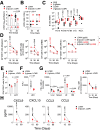
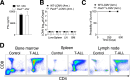
Viral infections pose a significant global burden. Host susceptibility to pathogens is determined by many factors including genetic variation that can lead to immunodeficient or dysregulated antiviral immune responses. Pax5 heterozygosity (Pax5-/+), resulting in reduced PAX5 levels in mice, mimics germline or somatic PAX5 dysregulation contributing to diseases such as childhood B-cell precursor acute lymphoblastic leukemia (B-ALL). In contrast to the well-characterized roles of PAX5 during early B-cell development, little is known about how Pax5 heterozygosity impacts antiviral responses. We infected Pax5-/+ mice with the noncytopathic Lymphocytic Choriomeningitis Virus (LCMV) and found that infection with the chronic Docile strain resulted in decreased survival of Pax5-/+ mice. While early adaptive CD8+ T-cell (CTL) immunity was robust in Pax5-/+ mice, LCMV-specific neutralizing antibody production was compromised leading to impaired long-term viral clearance and a pro-inflammatory milieu in the bone marrow (BM). Here we show that survival outcomes were improved upon prophylactic treatment with the β-glucan immune trainer through induction of heterologous protection against chronic infection. β-Glucan enhanced viral clearance, CTL immunity, neutralizing antibody production and reduced monocyte immunosuppression in multiple LCMV-resident host organs. New insight from this study will help design effective prophylactic treatment strategies against chronic viral infections, particularly in genetically predisposed susceptible hosts.
Keywords: Chronic Infection; LCMV; PAX5; Trained Immunity; β-glucan.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. PAL, KSL, HCX, and PP declare that they are involved in the development of LCMV for clinical application in oncology in cooperation with, as founders of, or as advisors to Abalos Therapeutics GmbH. The remaining authors declare no competing interests.
Figures








References
-
- Auer F, Ruschendorf F, Gombert M, Husemann P, Ginzel S, Izraeli S, Harit M, Weintraub M, Weinstein OY, Lerer I et al (2014) Inherited susceptibility to pre B-ALL caused by germline transmission of PAX5 c.547G>A. Leukemia 28:1136–1138 - PubMed
MeSH terms
Substances
Grants and funding
- 18R/2021,07/19/José Carreras Leukämie-Stiftung (DJCLS)
- TTU 07-711/Deutsches Zentrum für Infektionsforschung (DZIF)
- TTU 07.834/Deutsches Zentrum für Infektionsforschung (DZIF)
- 495318549/Deutsche Forschungsgemeinschaft (DFG)
- 547295412/Deutsche Forschungsgemeinschaft (DFG)
- EXC2151-390873048;DFG TRR237; DFG TRR259/Deutsche Forschungsgemeinschaft (DFG)
- LA2558/8-1,RTG1949/Deutsche Forschungsgemeinschaft (DFG)
- 85222/EC | European Research Council (ERC)
- 01KD2410A (EDI-4-ALL)/Bundesministerium für Bildung und Forschung (BMBF)
- 01KT2311;01KU2210/Bundesministerium für Bildung und Forschung (BMBF)
- 3618S32275; 3618S32275/Katharina-Hardt Stiftung
- 70114539/Deutsche Krebshilfe (German Cancer Aid)
- Priority Program Cancer Prevention-Graduate School/Deutsche Krebshilfe (German Cancer Aid)
- A2023/31/Deutsche Kinderkrebsstiftung (German Childhood Cancer Foundation)
LinkOut - more resources
Full Text Sources
Research Materials

