A B7H3-targeting antibody-drug conjugate in advanced solid tumors: a phase 1/1b trial
- PMID: 40082695
- PMCID: PMC12176648
- DOI: 10.1038/s41591-025-03600-2
A B7H3-targeting antibody-drug conjugate in advanced solid tumors: a phase 1/1b trial
Abstract
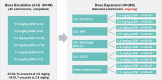
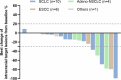
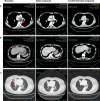
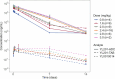
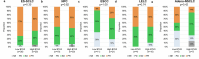
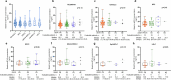
Antibody-drug conjugates (ADCs) have emerged as a transformative modality in the treatment of solid tumors. YL201, a novel B7H3-targeting ADC, leverages a tumor microenvironment activable linker-payload platform, coupled with a novel topoisomerase 1 inhibitor via a protease-cleavable linker. Here we report the findings from a large-scale, global, multicenter, phase 1 trial evaluating the safety, pharmacokinetics and preliminary efficacy of YL201 in patients with advanced solid tumors refractory to standard therapies. The trial included a dose-escalation part (phase 1) and a dose-expansion part (phase 1b). A total of 312 patients were enrolled across multiple tumor types, including extensive-stage small cell lung cancer (ES-SCLC), nasopharyngeal carcinoma (NPC), non-small cell lung cancer, esophageal squamous cell carcinoma and other solid tumors. The maximum tolerated dose was determined to be 2.8 mg kg-1, and the recommended expansion dose was selected as 2.0 mg kg-1 and 2.4 mg kg-1 every 3 weeks. The most common grade 3 or higher treatment-related adverse events included neutropenia (31.7%), leukopenia (29.5%) and anemia (25.0%). Only 4 cases of interstitial lung disease (1.3%) and 1 case of infusion reactions (0.3%) were observed. Encouraging anti-tumor activity was observed, particularly in patients with ES-SCLC (objective response rate (ORR), 63.9%), NPC (ORR, 48.6%), lung adenocarcinoma (ORR, 28.6%) and lymphoepithelioma-like carcinoma (ORR, 54.2%). No significant correlation between B7H3 membrane expression and the ORR was found. YL201 demonstrated an acceptable safety profile and a promising efficacy in heavily pretreated patients with advanced solid tumors, particularly in those with ES-SCLC, NPC or lymphoepithelioma-like carcinoma. Phase 3 clinical trials for patients with SCLC and NPC have already been initiated. ClinicalTrials.gov identifiers: NCT05434234 and NCT06057922 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: L.Z. reports receiving research support from AstraZeneca, Akeso Biopharma, Bristol Myers Squibb, Chia Tai Tianqing Pharmaceutical Group, Junshi Pharmaceuticals, QiLu Pharmaceutical and Pfizer. S.C., X.Z., W.L., J.C. and T.X. are employees of MediLink Therapeutics. The other authors declare no competing interests.
Figures




References
-
- Pulido, R., López, J. I. & Nunes-Xavier, C. E. B7-H3: a robust target for immunotherapy in prostate cancer. Trends Cancer10, 584–587 (2024). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

