Neutralization of acyl CoA binding protein (ACBP) for the experimental treatment of osteoarthritis
- PMID: 40082721
- PMCID: PMC12326017
- DOI: 10.1038/s41418-025-01474-y
Neutralization of acyl CoA binding protein (ACBP) for the experimental treatment of osteoarthritis
Abstract
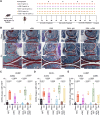
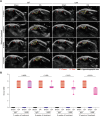
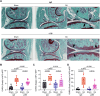
The plasma concentrations of acyl CoA binding protein (ACBP) encoded by the gene diazepam binding inhibitor (DBI) are increased in patients with severe osteoarthritis (OA). Here, we show that knee OA induces a surge in plasma ACBP/DBI in mice subjected to surgical destabilization of one hind limb. Knockout of the Dbi gene or intraperitoneal (i.p.) injection of a monoclonal antibody (mAb) neutralizing ACBP/DBI attenuates OA progression in this model, supporting a pathogenic role for ACBP/DBI in OA. Furthermore, anti-ACBP/DBI mAb was also effective against OA after its intraarticular (i.a.) injection, as monitored by sonography, revealing the capacity of ACBP/DBI to locally reduce knee inflammation over time. In addition, i.a. anti-ACBP/DBI mAb improved functional outcomes, as indicated by the reduced weight imbalance caused by OA. At the anatomopathological level, i.a. anti-ACBP/DBI mAb mitigated histological signs of joint destruction and synovial inflammation. Of note, i.a. anti-ACBP/DBI mAb blunted the OA-induced surge of plasma ACBP/DBI, as well as that of other inflammatory factors including interleukin-1α, interleukin-33, and tumor necrosis factor. These findings are potentially translatable to OA patients because joints from OA patients express both ACBP/DBI and its receptor GABAARγ2. Moreover, a novel mAb against ACBP/DBI recognizing an epitope conserved between human and mouse ACBP/DBI demonstrated similar efficacy in mitigating OA as an anti-mouse ACBP/DBI-only mAb. In conclusion, ACBP/DBI might constitute a promising therapeutic target for the treatment of OA.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: IM is a consultant for Osasuna Therapeutics. GK has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Sutro, Tollys, and Vascage. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics, and Therafast Bio. GK is in the scientific advisory boards of Hevolution, Institut Servier, Longevity Vision Funds, and Rejuveron Life Sciences. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis, and metabolic disorders. GK’s wife, Laurence Zitvogel, has held research contracts with Glaxo Smyth Kline, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9m, Tusk, and Roche, was on the Board of Directors of Transgene, is a co-founder of EverImmune, and holds patents covering the treatment of cancer and the therapeutic manipulation of the microbiota. GK’s brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim. The funders had no role in the design of the study; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79:819–28. - PubMed
-
- Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384:51–9. - PubMed
-
- Holden MA, Hattle M, Runhaar J, Riley RD, Healey EL, Quicke J, et al. Moderators of the effect of therapeutic exercise for knee and hip osteoarthritis: a systematic review and individual participant data meta-analysis. Lancet Rheumatol. 2023;5:e386–400. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

