A versatile toolbox for determining IRES activity in cells and embryonic tissues
- PMID: 40082722
- PMCID: PMC12048685
- DOI: 10.1038/s44318-025-00404-5
A versatile toolbox for determining IRES activity in cells and embryonic tissues
Abstract
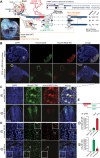
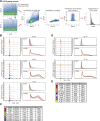
Widespread control of gene expression through translation has emerged as a key level of spatiotemporal regulation of protein expression. A prominent mechanism by which ribosomes can confer gene regulation is via internal ribosomal entry sites (IRESes), whose functions have however, remained difficult to rigorously characterize. Here we present a set of technologies in embryos and cells, including IRES-mediated translation of circular RNA (circRNA) reporters, single-molecule messenger (m)RNA isoform imaging, PacBio long-read sequencing, and isoform-sensitive mRNA quantification along polysome profiles as a new toolbox for understanding IRES regulation. Using these techniques, we investigate a broad range of cellular IRES RNA elements including Hox IRESes. We show IRES-dependent translation in circRNAs, as well as the relative expression, localization, and translation of an IRES-containing mRNA isoform in specific embryonic tissues. We thereby provide a new resource of technologies to elucidate the roles of versatile IRES elements in gene regulation and embryonic development.
Keywords: Embryo Development; Internal Ribosome Entry Site; cellular IRES; mRNA Isoforms; mRNA Translation Regulation.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. KL and MB are inventors of patents related to the Hoxa9 P4 stem-loop and RNA therapeutics and their various uses. PK, MB, and KL are inventors on a patent related to IRES-like elements in circRNA reporters and RNA therapeutics and their various uses. The remaining authors declare no competing interests.
Figures









References
-
- Brito Querido J, Díaz-López I, Ramakrishnan V (2023) The molecular basis of translation initiation and its regulation in eukaryotes. Nat Rev Mol Cell Biol 25:168–186 - PubMed
-
- Burke AC, Nelson CE, Morgan BA, Tabin C (1995) Hox genes and the evolution of vertebrate axial morphology. Development 121:333–346 - PubMed
-
- Burke TW, Kadonaga JT (1996) Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes Dev 10:711–724 - PubMed
MeSH terms
Substances
Grants and funding
- 216372545/Deutsche Forschungsgemeinschaft (DFG)
- R01-GM129325/HHS | National Institutes of Health (NIH)
- RC22025/Rosetta Commons Mini Grant
- start-up funds/University of Bonn, University Clinic Bonn
- Life and Health Research Prize 2024/University of Bonn, TRA
- Graduate Research Fellowship DGE-114747/National Science Foundation (NSF)
- National Science Scholarship (Ph.D.)/Agency for Science, Technology and Research (A*STAR)
- EXC2151/Deutsche Forschungsgemeinschaft (DFG)
- R01 HD086634/HD/NICHD NIH HHS/United States
- R01 GM129325/GM/NIGMS NIH HHS/United States
- 5R01HD086634/HHS | National Institutes of Health (NIH)
- PhD Position/University of Bonn, STEP
- P50 training grant/HHS | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

