Metrnl ameliorates myocardial ischemia-reperfusion injury by activating AMPK-mediated M2 macrophage polarization
- PMID: 40082768
- PMCID: PMC11907862
- DOI: 10.1186/s10020-025-01150-4
Metrnl ameliorates myocardial ischemia-reperfusion injury by activating AMPK-mediated M2 macrophage polarization
Abstract
Background: Meteorin-like hormone (Metrnl) is prominently expressed in activated M2 macrophages and has demonstrated potential therapeutic effects in a range of cardiovascular diseases by modulating inflammatory responses. Nevertheless, its precise role and the underlying mechanisms in myocardial ischemia/reperfusion injury (MI/RI) are not fully understood. This study examined whether Metrnl can mitigate MI/RI through the AMPK-mediated polarization of M2 macrophages.
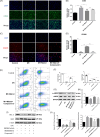
Methods: In vivo, adeno-associated virus 9 containing the F4/80 promoter (AAV9-F4/80) was utilized to overexpress Metrnl in mouse cardiac macrophages before MI/RI surgery. In vitro, mouse bone marrow-derived macrophages (BMDMs) were treated with recombinant protein Metrnl, and the human cardiomyocyte cell line AC16 was subjected to hypoxia/reoxygenation (H/R) after co-culture with the supernatant of these macrophages. Cardiac function was assessed via echocardiography, H&E staining, and Evans blue-TTC staining. Inflammatory infiltration was evaluated by RT-qPCR and ELISA, apoptosis by Western blotting and TUNEL staining, and macrophage polarization by immunofluorescence staining and flow cytometry.
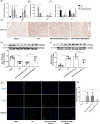
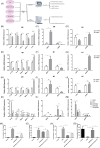
Results: In vivo, Metrnl overexpression in cardiac macrophages significantly attenuated MI/RI, as evidenced by reduced myocardial infarct size, enhancement of cardiac function, diminished inflammatory cell infiltration, and decreased cardiomyocyte apoptosis. Furthermore, Metrnl overexpression promoted M1 to M2 macrophage polarization. In vitro, BMDMs treated with Metrnl shifted towards M2 polarization, characterized by decreased expression of inflammatory cytokines (IL-1β, MCP-1, TNF-α) and increased expression of the anti-inflammatory cytokine IL-10. Additionally, supernatant from Metrnl-treated macrophages protected AC16 cells from apoptosis under H/R conditions, as evidenced by decreased BAX expression and increased BCL-2 expression. However, these effects of Metrnl were inhibited by the AMPK inhibitor Compound C.
Conclusions: Metrnl alleviates MI/RI by activating AMPK-mediated M2 macrophage polarization to attenuate inflammatory response and cardiomyocyte apoptosis. This study highlights the therapeutic potential of Metrnl in MI/RI, and identifies it as a promising target for the treatment of ischemic heart disease.
Keywords: AMPK; Apoptosis; Inflammation; Macrophage polarization; Metrnl; Myocardial ischemia–reperfusion injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Animal experiments in this study were approved by the Animal Care and Welfare Committee of Guangxi Medical University (202111022). Consent for publication: All authors agree with the manuscript content. Competing interests: The authors declare no competing interests.
Figures



References
-
- Algoet M, et al. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc Med. 2023;33(6):357–66. - PubMed
-
- Chen X, et al. Protective role of the novel cytokine Metrnl/ interleukin-41 in host immunity defense during sepsis by promoting macrophage recruitment and modulating Treg/Th17 immune cell balance. Clin Immunol. 2023a;254:109690. - PubMed
MeSH terms
Substances
Grants and funding
- YCSW2023243/Innovation Project of Guangxi Graduate Education
- GXXNXG202205/Open subject of Guangxi Key Laboratory of Precision Medicine for Cardiovascular and Cerebrovascular Disease Prevention and Treatment
- 82070279/National Natural Science Foundation of China
- 19-245-34/Guangxi Key Laboratory of Precision Medicine in Cardio-Cerebrovascular Diseases Control and Prevention
- AD17129014/Guangxi Clinical Research Center for Cardio-Cerebrovascular Diseases
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

