Effect of Helicobacter pylori infection on survival outcomes of patients undergoing radical gastrectomy after neoadjuvant chemotherapy: a multicenter study in China
- PMID: 40082850
- PMCID: PMC11907980
- DOI: 10.1186/s12885-025-13840-7
Effect of Helicobacter pylori infection on survival outcomes of patients undergoing radical gastrectomy after neoadjuvant chemotherapy: a multicenter study in China
Abstract
Background: Neoadjuvant chemotherapy (NAC) has been confirmed to improve the prognosis of patients with advanced gastric cancer (AGC). However, no study has investigated whether Helicobacter pylori (HP) infection affects the postoperative survival of patients who receive NAC.
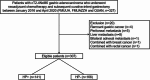
Methods: This retrospective cohort study included 307 patients with AGC who underwent laparoscopic radical gastrectomy after NAC at three hospitals in China between January 1, 2016, and April 31, 2020. Cox regression was used to assess prognostic factors for survival. Kaplan-Meier was used for survival analysis.
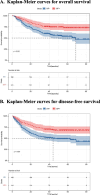
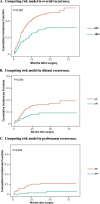
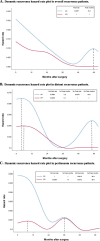
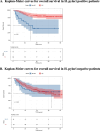
Results: The HP + and the HP- group included 141 and 166 cases. The 3-year overall survival (OS) and disease-free survival (DFS) of the HP + group were significantly better than the HP- group (3-year OS: 75.9% vs. 60.2%, 3-year DFS: 70.2% vs. 52.3%; All P < 0.001). For the HP + group, ypTNM Stage III (HR, 4.00; 95% CI, 1.11-14.39; P = 0.034), NAC ≥ 4 cycles (HR, 0.43; 95% CI, 0.20-0.90; P = 0.026), and adjuvant chemotherapy (AC) ≥ 4 cycles (HR, 0.20; 95% CI, 0.09-0.48; P < 0.001) are independent prognostic factors for OS. In the cohort of HP + patients who received ≥ 4 cycles of NAC, the prognosis of patients who received ≥ 4 cycles of AC after surgery was better than that of patients who received < 4 cycles of AC (3-year OS: 92.5% vs 71.4%; P = 0.042).
Conclusions: Following NAC, HP + patients with AGC exhibit better prognosis than that of HP- counterparts. For potentially resectable HP + AGC patients, radical surgery following ≥ 4 cycles of NAC with ≥ 4 cycles of sequential AC might be recommended to improve survival.
Keywords: Helicobacter pylori; Gastrectomy; Gastric cancer; Neoadjuvant chemotherapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol has been reviewed and approved by the Ethics Committees of Fujian Medical University Union Hospital, Fujian Medical University Affiliated Zhangzhou Hospital, and Qinghai University Affiliated Hospital (Number of IRB: 2024KY090), in accordance with the ethical regulations for biomedical research in the People's Republic of China. All participants from the three hospitals signed written informed consent forms prior to their inclusion in the study. The consent forms clearly included the following elements: 1) the purpose, methods, and expected duration of the study; 2) foreseeable risks and potential benefits; 3) the voluntary nature of participation and the right to withdraw unconditionally; and 4) data anonymization and confidentiality measures. For patients unable to provide direct consent (such as those in a coma), we obtained proxy consent from legal guardians or close relatives. All patient data are stored in a de-identified coded format, with access to the original data restricted to core members of the research team. When the study results are published, no identifiable personal information will be included. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660. - PubMed
-
- Songun I, Putter H, Kranenbarg EMK, Sasako M, van de Velde CJH. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11(5):439–49. 10.1016/S1470-2045(10)70070-X. - PubMed
-
- Ajani JA, D’Amico TA, Almhanna K, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(10):1286–312. 10.6004/jnccn.2016.0137. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

