ADT-1004: a first-in-class, oral pan-RAS inhibitor with robust antitumor activity in preclinical models of pancreatic ductal adenocarcinoma
- PMID: 40082968
- PMCID: PMC11905721
- DOI: 10.1186/s12943-025-02288-9
ADT-1004: a first-in-class, oral pan-RAS inhibitor with robust antitumor activity in preclinical models of pancreatic ductal adenocarcinoma
Abstract
Background: Oncogenic KRAS mutations occur in nearly, 90% of patients with pancreatic ductal adenocarcinoma (PDAC). Targeting KRAS has been complicated by mutational heterogeneity and rapid resistance. We developed a novel pan-RAS inhibitor, ADT-1004 (an oral prodrug of ADT-007) and evaluated antitumor activity in murine and human PDAC models.
Methodology: Murine PDAC cells with KRASG12D mutation (KPC-luc or 2838c3-luc) were orthotopically implanted into the pancreas of C57BL/6J mice, and four PDX PDAC tumors with KRAS mutations were implanted subcutaneously in NSG mice. To assess potential to overcome RAS inhibitor resistance, parental and resistant MIA PaCa-2 PDAC cells (KRASG12C mutation) were implanted subcutaneously. Subcutaneously implanted RASWT BxPC-3 cells were used to assess the selectivity of ADT-1004.
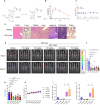
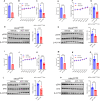
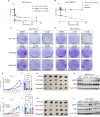
Results: ADT-1004 potently blocked tumor growth and RAS activation in mouse PDAC models without discernable toxicity with target engagement and reduced activated RAS and ERK phosphorylation. In addition, ADT-1004 suppressed tumor growth in PDX PDAC models with KRASG12D, KRASG12V, KRASG12C, or KRASG13Q mutations and increased CD4+ and CD8+ T cells in the TME consistent with exhaustion and increased MHCII+ M1 macrophage and dendritic cells. ADT-1004 demonstrated superior efficacy over sotorasib and adagrasib in tumor models resistant to these KRASG12C inhibitors and MRTX1133 resistant KRASG12D mutant cells. As evidence of selectivity for tumors with mutant KRAS, ADT-1004 did not impact the growth of tumors from RASWT PDAC cells.
Conclusion/significance: ADT-1004 has strong antitumor activity in aggressive and clinically relevant PDAC models with unique selectivity to block RAS-mediated signaling in RAS mutant cells. As a pan-RAS inhibitor, ADT-1004 has broad activity and potential efficacy advantages over allele-specific KRAS inhibitors. These findings support clinical trials of ADT-1004 for KRAS mutant PDAC.
Keywords: KRAS; Pancreatic ductal adenocarcinoma; RAS-driven malignancies; Tumor immune microenvironment; pan-RAS inhibitor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Not applicable. Consent to participate: Not applicable. Consent to publish: Not applicable. Competing interests: The authors declare no competing interests.
Figures




Update of
-
ADT-1004: A First-in-Class, Orally Bioavailable Selective pan-RAS Inhibitor for Pancreatic Ductal Adenocarcinoma.bioRxiv [Preprint]. 2024 Oct 8:2024.10.04.616725. doi: 10.1101/2024.10.04.616725. bioRxiv. 2024. Update in: Mol Cancer. 2025 Mar 13;24(1):76. doi: 10.1186/s12943-025-02288-9. PMID: 39416034 Free PMC article. Updated. Preprint.
References
-
- Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008–20. - PubMed
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Jiang Y, Sohal DP. Pancreatic adenocarcinoma management. JCO Oncol Pract. 2023;19(1):19–32. - PubMed
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. Cancer J Clin. 2024;74(1). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

