T cell receptor activation contributes to brain damage after intracerebral hemorrhage in mice
- PMID: 40082981
- PMCID: PMC11905663
- DOI: 10.1186/s12974-025-03402-w
T cell receptor activation contributes to brain damage after intracerebral hemorrhage in mice
Abstract
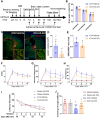
Background: Our previous studies demonstrated that activated T cells accumulate in perihematomal regions following intracerebral hemorrhage (ICH) and exacerbate hemorrhagic brain injury. In the present study, we aimed to explore the mechanisms underlying brain-infiltrating T cell activation and the associated pathophysiological effects in neurological outcomes following ICH.
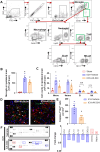
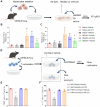
Methods: We employed standardized collagenase injection-induced and autologous blood injection models of ICH in male C57BL/6J mice. T cell receptor (TCR) activation, immune cell infiltration, and cytokine production were quantified through immunostaining, flow cytometry, and cytokine arrays at 1- and 3-days post-ICH. Brain edema volume was measured at 3 days post-ICH and neurobehavioral assessments were conducted up to 14 days post-ICH. Pharmacological inhibition of TCR activation was achieved using the TCR-specific inhibitor AX-024, administered intraperitoneally at a dosage of 10 mg/kg 1-hour post-ICH.
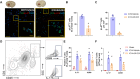
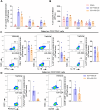
Results: Flow cytometry and immunostaining detected TCR activation of brain-infiltrating T cells. Specific TCR activation inhibitor AX-024 administration markedly reduced TCR activation and the production of pro-inflammatory cytokines in the brain at 1- and 3-days post-ICH. Moreover, AX-024 administration led to a significant reduction in the infiltration of other leukocyte populations, and significantly reduced brain edema while improved long-term sensorimotor and cognitive outcomes up to 14 days post-ICH.
Discussion: Our findings underscore the critical role of TCR activation in the mobilization and activation of brain-infiltrating T cells post-ICH. Inhibition of TCR activation via AX-024 administration might be developed as a promising therapeutic strategy to improve neurological outcomes following ICH. However, further research is necessary to thoroughly explore the complex pathophysiological processes involved.
Keywords: Inflammation; Intracerebral hemorrhage; Leukocyte; T cell activation; T-cell receptor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: All animal experiments were performed with ethics approval (protocol ID: 2118) from the Institutional Animal Care and Use Committee (IACUC) of Tulane University. Competing interests: The authors declare no competing interests.
Figures

References
-
- Sun Q, et al. Neurovascular units and Neural-Glia networks in intracerebral hemorrhage: from mechanisms to translation. Transl Stroke Res. 2021;12(3):447–60. - PubMed
-
- Hemphill JC 3, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American heart association/american stroke association. Stroke. 2015;46(7):2032–60. - PubMed
-
- Mracsko E, et al. Leukocyte invasion of the brain after experimental intracerebral hemorrhage in mice. Stroke. 2014;45(7):2107–14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

