PD-L1+ CD49f+ CD133+ Circulating tumor cells predict outcome of patients with vulvar or cervical cancer after radio- and chemoradiotherapy
- PMID: 40083005
- PMCID: PMC11908062
- DOI: 10.1186/s12967-025-06277-w
PD-L1+ CD49f+ CD133+ Circulating tumor cells predict outcome of patients with vulvar or cervical cancer after radio- and chemoradiotherapy
Abstract
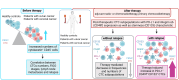
Background: Monitoring individual therapy responses of patients with cancer represents a major clinical challenge providing the basis to early identify metastases and cancer relapse. We previously demonstrated that radio- or chemoradiotherapy affects the systemic cellular milieu of patients with vulvar or cervical cancer and creates individual post-therapeutic environments associated with cancer relapse. Circulating tumor cells (CTCs) in the systemic milieu are related to metastases and relapse; however, their quantitative and phenotypic characteristics during therapy of patients with vulvar and cervical cancer are still unknown.
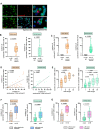
Methods: In this prospective, longitudinal study, we verified the presence of CTCs via immunofluorescence and systemically characterized CTCs by flow cytometry from the blood of 40 patients with vulvar and 115 patients with cervical cancer receiving surgery, adjuvant radiotherapy (aRT), chemoradiotherapy (aCRT) or primary chemoradiotherapy (pCRT) and linked the presence of different CTC subpopulations with individual outcome of disease.
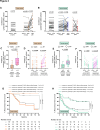
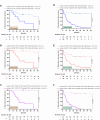
Results: Pre-therapeutic cytokeratin+ CD45- CTC numbers significantly correlated with tumor FIGO stages, lymph node metastases and relapse. While surgery only did not significantly alter CTC occurrence, aRT and aCRT as well as pCRT differentially decreased or increased CTCs in patients with both tumor entities compared to baseline levels. Therapy-mediated increased CTC numbers were directly linked with subsequent cancer recurrence on follow-up. Phenotypic characterization of CTCs revealed enhanced expression of the stem cell marker CD133 as well as the integrin α6 (CD49f) after aRT, aCRT and pCRT. Furthermore, the aRT, aCRT and pCRT cohorts exhibited increased proportions of Programmed Cell Death Protein Ligand (PD-L1) expressing cells among post-therapeutic CTCs. Notably, post-therapeutic PD-L1+ CD49f+ CD133+ numbers ≥ 5/ml in patients with vulvar cancer and ≥ 2/ml in patients with cervical cancer were associated with reduced recurrence-free survival on follow-up.
Conclusion: Our study unravels individual therapy-induced changes in CTC phenotypic characteristics and occurrence in the patients' blood and their association with cancer relapse. Our results may help to explain differences in the individual courses of disease of patients with vulvar and cervical cancer and suggest PD-L1, CD49f and CD133 as targets for immunotherapy in vulvar and cervical cancer.
Keywords: Cervical cancer; Chemoradiotherapy; Circulating tumor cells; Radiotherapy; Vulvar cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study has been conducted according to Declaration of Helsinki principles. Usage of blood samples of patients with vulvar and cervical cancer and healthy controls were approved by the Ethics Committees of the Saarland Ärztekammer (Saarbrücken, Germany; 98/17 and 121/21). Written informed consent was provided by all study participants. Consent for publication: Written informed consent was obtained from the participants to use blinded individual details for research purposes and publications. Competing interests: G.O. received grants form AstraZeneca (Cambridge, UK), Medconcept (Neustadt an der Weinstraße, Germany), University Medical Center Freiburg (Germany), Der PRIVATARZT Gynäkologie (MiM Verlagsgesellschaft mbH, Neu-Isenburg, Germany) and RG Ärztefortbildung GmbH, membership of DGGG (Berlin, Germany), AGE (Buchholz, Germany), and AGEM (Berlin, Germany) and scientific colloboration with Karl Storz (Tuttlingen, Germany) outside the submitted work. The other authors declare no potential conflicts of interest.
Figures


References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. - PubMed
-
- Rogers LJ, Cuello MA. Cancer of the vulva. Int J Gynaecol Obstet. 2018;143(Suppl 2):4–13. - PubMed
-
- de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–15. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

