Lungfish-like antero-labial tooth addition and amphibian-like enameloid-enamel transition in the coronoid of a Devonian stem actinopterygian
- PMID: 40083060
- PMCID: PMC12397074
- DOI: 10.1111/joa.14240
Lungfish-like antero-labial tooth addition and amphibian-like enameloid-enamel transition in the coronoid of a Devonian stem actinopterygian
Abstract
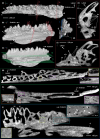
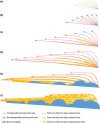
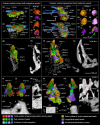
New teeth are predominantly initiated lingually or postero-lingually to the old teeth in vertebrates. Osteichthyan dentitions typically consist of linear rows of shedding teeth, but internal to the marginal jawbones osteichthyans primitively have an extra dental arcade, in which teeth are sometimes spread out into a field and not organized in rows. The tooth plates of lungfish are specialized from the jawbones of the inner dental arcade, but the teeth are arranged in radial tooth rows with new teeth added at the anterior and labial end of the rows and without shedding the old teeth, distinct from other osteichthyan dentitions. Actinopterygian teeth can be recognized by a cap of enameloid, while sarcopterygian teeth are only coated by enamel. An enameloid cap is also borne by the unicuspid larval teeth in some amphibians, but it is covered by enamel and eventually disappears in the bicuspid adult teeth. In early osteichthyans, old teeth are often not completely resorbed and shed, and the overlapping relationship of their remnants buried in the bone records the sequence of developmental events. Using synchrotron microtomography, this ontogenetic record of a coronoid tooth field of a Devonian stem actinopterygian is visualized in 3D. As a component of the inner dental arcade, the coronoid displays initial radial non-shedding tooth rows followed by radial shedding tooth rows that are later transformed into linear shedding tooth rows. The teeth are always added antero-labially and replaced labially to keep pace with the labial bone apposition and lingual bone remodeling, which causes the shift of the tooth competent zone. These provide a clue to the evolution of the radial non-shedding dentition with antero-labial tooth addition in lungfish. The tooth patterning process suggests that the superficial disorder of the tooth field is an epiphenomenon of the ever-changing local developing environment of each tooth bud: due to the retention of old tooth bases, a tooth position that has been replaced in place can at some point drift to a site between the adjacent tooth positions, splitting or merging, and then continue being replaced in situ. Primary teeth are capped by enameloid, but replacement teeth bear enamel crests without an enameloid cap. This demonstrates that the transition from enameloid capping to enamel coating through tooth replacement can happen in actinopterygians too, as one of the mechanisms for a dentition to change tooth shape. All these unexpected observations indicate that, during ontogeny, the states of dental characters, such as lingual/labial tooth initiation, linear/radial tooth rows, in situ/cross-position tooth replacement and enameloid/enamel, can be switched and the capacity to produce these characters can be suspended or reactivated; the tremendous dental diversity can thus be attributed to the manipulation in time and space of relatively few dental developmental processes.
Keywords: Radial‐linear tooth row transition; cross‐position tooth replacement; dental patterning; dentine‐dentine attachment; heterochrony; mineralized tissue transition; shift of dental competent zone; tooth shape transition.
© 2025 The Author(s). Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Figures




References
-
- Ahlberg, P.E. & Clack, J. (1998) Lower jaws, lower tetrapods – a review based on the Devonian genus Acanthostega . Transactions of the Royal Society of Edinburgh: Earth Sciences, 89, 11–46.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

